We investigated the psychostimulant, rewarding, and anxiolytic-like effects of pulegone. Possible interactions between pulegone and menthol concerning their psychostimulant effect were also analyzed. General mouse activity after pulegone treatment, and the interacitons between pulegone and menthol, were determined in the open field. The anxiolytic-like activity, motor coordination and strength force were evaluated using the elevated plus maze (EPM), rotarod test and grasping test, respectively. The motivational properties of pulegone were evaluated by pairing the drug effects on the mice with the least preferred compartment (previously determined) of a conditioned place preference (CPP) apparatus. Pulegone increased mouse locomotor activity and immobilization time. Verapamil, but not diltiazem, haloperidol or picrotoxin, decreased the psychostimulation induced by pulegone. Pulegone also decreased grooming and rearing behaviors and caused motor incoordination and weakness at high doses. Pulegone increased the time spent by mice in the open arms of the EPM, and flumazenil pre-treatment did not alter this effect. Pulegone either produced no CPP or induced conditioned place aversion. The changes in mouse ambulatory activity caused by the association of pulegone with menthol were either lower than those predicted by the theoretical curve or not different from the predicted values. Therefore, pulegone induces a verapamil-sensitive psychostimulant effect that appears to independ on the opening of L-type calcium channels. Pulegone has negative reinforcing properties and seems to possess anxiolytic-like actions unrelated to the benzodiazepine site of the γ-aminobutyric acid type A (GABAA) receptor. Finally, pulegone might act in an addictive or synergic way with menthol.
Pulegone is a monoterpenic compound found in many plants. This compound is found in Mentha piperita L. (peppermint) essential oil and is the main constituent of Mentha pulegium L. (pennyroyal) and Ziziphora clinopodioides (blue mint bush) essential oils (60–90%).1–4) M. piperita, M. pulegium and Z. clinopodioides are pharmacologically and toxicologically important worldwide.1,5–8) Peppermint oil has been used in alternative medicine practices, such as aromatherapy, and has been claimed to have beneficial effects in the treatment of mental diseases.7,8) Peppermint has also been found to improve cognitive performance and increase alertness9); moreover, a near fatal case due to ingestion of toxic dose of peppermint oil was recently reported.6) Pennyroyal has been used to treat dizziness, as an anticonvulsive and as a sedative and is still sometimes used as an abortifacient.10,11) Blue mint bush has been used as a sedative and in cooking and to prevent food decay.1) Additionally, another source of human exposure to pulegone is the consumption of beverage flavoring and confections.12)
Despite its use in traditional medicine, pennyroyal oil is known to be extremely hepatotoxic and neurotoxic. Pulegone causes extensive liver damage in laboratory animals,13) and human intoxication with pennyroyal oil (or with mint tea containing this oil) is associated with the loss of renal function, hepatotoxicity, dizziness, epileptic encephalopathy and, in severe cases, death.14) Although the hepatotoxic effects of pulegone have been extensively demonstrated and studied, the behavioral effects of this terpenic compound have only recently gained attention in the field.15,16) Further investigation of the behavioral effects of pulegone could increase our understanding of the pharmacology and toxicology of natural products containing pulegone. Umezu16) reported that pulegone increases mouse locomotor activity, and this effect was sensitive to dopamine receptor antagonists, suggesting that dopamine was involved in the ambulation promoted by pulegone. It is well established that the mesolimbic dopaminergic system is involved in the rewarding and psychostimulant effects of drugs of abuse and that this system has important functions in the behaviors that are reinforced by drugs.17,18) Therefore, we evaluated the psychostimulant effect of pulegone and tested the hypothesis that this compound has rewarding properties in addition to being a psychostimulant.
Moreover, the reported sensitivity of motor stimulation that is induced by pulegone to dopamine antagonists is similar to that of classical psychostimulants, such as cocaine and amphetamines.19–21) However, pulegone-induced psychostimulation is associated with ataxia,16) an effect that is more closely associated with low doses of classical depressants, such as ethanol,22) than with classical stimulants, which usually cause stereotypy and not ataxia. Therefore, we investigated the pharmacological similarities between pulegone and classical depressants; to this purpose, we studied the sensitivity of pulegone-induced psychostimulation to drugs that have been shown to inhibit ethanol-induced increases in locomotor activity, but not the increases in locomotor activity that are induced by classical stimulants.22,23) Additionally, because natural products that contain a large amount of pulegone have been used as sedatives,1) we also investigated the possible anxiolytic-like actions of this substance. Finally, considering that essential oils used as alternative medicines are complex mixtures of different terpenic compounds that could interact, we investigated the possible pharmacological interactions between pulegone and menthol, another important major constituent of several essential oils.4)
The experiments were performed using male Swiss mice (35–45 g), and all the protocols were reviewed and approved by the Ethics Committee on Animal Use of the Federal University of Uberlândia (CEUA/UFU process n° 082/10, 116/11, 024/12 and addendum 236/13). The animals were housed in a room at 21°C with 12 h light/dark cycles and were provided free access to tap water and standard chow.
Open Field TestThe general activity of the animals was evaluated 10 min after treatment with olive oil (control; 0.2 mL/animal, intraperitoneally (i.p.)) or pulegone (100, 200, 400 or 800 mg/kg, i.p., dissolved in olive oil) through direct observation in an open field exploration apparatus. The apparatus consisted of a circular wood arena that was 40 cm in diameter. The animals were placed in the center of the arena and were observed for 5 min. During this time, the spontaneous ambulation (number of segments crossed by the animal with all four legs), the rearing (number of times that the animal stood on its hind legs), and the amount of time(s) spent immobilized (totally frozen) or grooming were recorded. The experiments were conducted between 13:00 and 18:00 h. Before introducing each animal into the arena, the apparatus was washed with a 5% (v/v) ethanol–water solution to avoid possible bias due to odor trails left by previous animals. Each animal was evaluated only once in the open field. The time after drug administration that was used to analyze the mouse behavior was chosen based on the previously reported peak of pulegone-induced psychostimulation.16) The effects of pulegone (400 mg/kg, i.p.) on mouse locomotor activity were also analyzed in animals that had been pre-treated with haloperidol (a dopamine D2 receptor antagonist; 0.01 mg/kg or 0.125 mg/kg, i.p.; 30 min prior to pulegone treatment), picrotoxin (a γ-aminobutyric acid type A (GABAA) receptor blocker; 0.25 mg/kg, i.p.; 15 min prior to pulegone treatment), verapamil or diltiazem (L-type voltage-dependent calcium channel blockers; 15 mg/kg and 20 mg/kg, respectively, i.p., 30 min prior to pulegone treatment). The doses of the antagonist/blockers and the amount of time prior to pulegone treatment that the antagonist/blockers were injected were chosen based on data from the literature on the efficacy of the posologic regimes.16,19,22,23) To investigate the pharmacological interactions between pulegone and menthol concerning the increase in locomotor activity, the animals were treated with pulegone (100 or 200 mg/kg, i.p.), menthol (100 or 200 mg/kg, i.p.) or a combination of both (pulegone 100 mg/kg+menthol 100 mg/kg; pulegone 100 mg/kg+menthol 200 mg/kg; pulegone 200 mg/kg+menthol 100 mg/kg and pulegone 200 mg/kg+menthol 200 mg/kg, i.p.). The mean amount of spontaneous locomotor activity of the group treated with olive oil (control) was used as the basal ambulation (BA); the differences between the basal value and the ambulation observed in mice treated with pulegone and menthol were then calculated (ambulation observed in a specific treated group—BA=Δ effect of the specific treatment). The Δ mean of the effect produced by the administration of pulegone or menthol alone was determined, and the equations used to construct the theoretical curves for each specific association were as follows: Δ mean of pulegone 100 mg/kg+Δ mean of menthol 100 mg/kg=Δ mean theoretical value of this association, Δ mean of pulegone 100 mg/kg+Δ mean of menthol 200 mg/kg=theoretical valued of this association, and so on. The effect of administering pulegone with menthol in a specific association was then compared with the theoretical value.
Rotarod TestImmediately after evaluation in the open field, motor coordination and balance were tested using an accelerating rotarod as previously described by Pelkonen and Yavich,24) with modification. The animals were allowed to become accustomed to the apparatus for 1 min before testing (adaption phase), which included 30 s on a motionless bar and 30 s on a bar that was rotating at a minimal speed (16 rpm). After this period of adaptation, the apparatus was set to accelerate (from 16 to 37 rpm), and the timing began. The amount of time that the animals were able to run on the rotating bar without falling off of the rod was recorded. If the animal fell from the bar during the adaption phase, it was put back on the bar, and the procedure was reinitiated. If necessary, the animal was reintroduced to the bar five consecutive times. If the animal fell after the fifth time, the animal was removed from the rod, and the performance on the apparatus was recorded as 0 s. The maximum time the animals were allowed to remain on the rod was 360 s.
Grasping TestThis experiment was conducted as previously described25) with some modifications. After evaluation on the rotarod, mice were gently lifted by the tail and allowed to grasp a bar connected to an electronic balance with adhesive tape. While grasping the bar, the mice continued to be lifted by the tail with increasing firmness until their grip loosened. At that precise moment, the negative value shown by the balance was recorded. This procedure was repeated three times, and the mean of the measures was used as the grasping strength (g).
Conditioning Place Preference (CPP) TestFor the CPP test, we used two identical Plexiglas three-chamber boxes with two equal-sized compartments (20×20 cm) separated by a neutral grey chamber (20×7.5 cm).26) The boxes were separated by two sliding doors. The two large compartments had different colored walls (black-and-white striped or only white) and different flooring (sheet metal or stiff wire mesh). The black-and-white striped wall chamber had a stiff wire mesh floor, and the white wall chamber had a sheet metal floor. The experimental procedure consisted of three phases, as previously described,27) with the following modifications: i) preconditioning—in this phase, the animals were initially placed in the neutral grey chamber, and the doors were removed. Each mouse was allowed to explore the apparatus for 25 min. This procedure was repeated over 3 consecutive days. The time spent in each compartment was recorded on the third day to determine the naturally least preferred compartment for each animal. ii) conditioning phase—animals were injected with olive oil or pulegone (100 or 200 mg/kg, i.p.) on alternate days for 6 consecutive days (i.e., from day 4 to day 9, with animals receiving olive oil on day 4) and confined in one of the two compartments for 25 min as follows: pulegone-treated mice were confined in the least preferred compartment when receiving pulegone and in the most preferred compartment when receiving olive oil. This procedure was chosen because it has been reported that, for some drugs, a CPP could be detected only when the drug is paired with the least preferred side of the CPP apparatus.28) The control groups received only olive oil independent of confinement. iii) post-conditioning (test)—on day 10, the mice were placed in the neutral grey chamber, the doors were removed, and the animals were allowed to freely move inside the apparatus. The time spent in each compartment was recorded for 25 min without exposure to drug. To evaluate the CPP, the values for the time spent by each animal in the least preferred compartment during pre-conditioning and post-conditioning were measured, and the percentages of the post-conditioning time were calculated in relation to the pre-conditioning time (i.e., time spent in the least preferred compartment on day 10/time on day 3×100). Increases and decreases in this parameter were defined as the CPP and the conditioned place aversion (CPA), respectively. The doses of 400 and 800 mg/kg of pulegone were not tested in the CPP paradigm because they were extremely toxic (mortality within 24 h: 60% and 100%, respectively).
Elevated Plus Maze (EPM) TestThe EPM consisted of two open arms (26.5 cm) and two closed arms (26.5 cm), with the open pair perpendicular to the closed pair. The arms were connected by a central area (14 cm). The maze was made of wood and was elevated 49.0 cm above the floor. Ten minutes after olive oil (0.2 mL/animal, i.p.), pulegone (100, 200 or 400 mg/kg, i.p.), or diazepam (1.0 mg/kg, i.p.) treatment, the mice were individually placed at the center of the EPM and observed for 5 min. The number of entries and the time (in s) spent by the animals in the open arms were recorded. Data were expressed as entries in the open arms/total entries (%) and time spent in the open arms (%). To investigate the possible mechanism underlying the anxiolytic-like activities of pulegone, the animals were intraperitoneally pre-treated with flumazenil (2.5 mg/kg; 30 min before the administration of pulegone, 400 mg/kg, i.p.), which is an antagonist of GABAA/benzodiazepine receptors. The 800 mg/kg dose of pulegone was not tested in the EPM because administration of this dose produced a prominent increase in immobilization time, which was linked to profound modifications in motor coordination and force and could affect mouse performance on this apparatus; this could cause the data to be difficult to interpret.
DrugsR-(+)-Pulegone, picrotoxin and verapamil were purchased from Sigma-Aldrich (U.S.A.), haloperidol was purchased from TEUTO (Brazil), flumazenil was purchased from Eurofarma (Brazil), diazepam was purchased from Laboratório Santisa (Brazil), diltiazem was gently gift by Laboratórios Baldacci S/A (Brazil) and the olive oil was purchased from Sandeleh Alimentos LTDA (Brazil).
Statistical AnalysisData are given as the mean±S.E.M. for the indicated number of independent experiments. CPP and data from the experiments conducted with the association of pulegone and menthol were analyzed with a one-sample t-test using the value of 100% as the control or the values obtained in the theoretical curves. Data from other behavioral experiments were compared using one-way ANOVA with the Newman–Keuls post-hoc test. The statistical analyses were performed using GraphPad® Software, and statistical significance was accepted at p<0.05.
Pulegone treatment increased the ambulatory activity of mice in a dose-dependent and bell-shaped manner (Fig. 1A). Haloperidol administration did not suppress the psychostimulant effect of pulegone (400 mg/kg) in any of the tested doses (Fig. 2A); moreover, haloperidol decreased ambulatory activity only at a dose of 0.125 mg/kg (Fig. 2A). Administration of verapamil, but not diltiazem or picrotoxin, significantly decreased the psychostimulant effect of pulegone (Figs. 2B, C). Verapamil, diltiazem or picrotoxin alone did not alter the locomotor activity of the mice (Figs. 2B, C). Pulegone increased the immobilization time at the 100 and 800 mg/kg doses (Fig. 1D) and decreased rearing at the 800 mg/kg dose (Fig. 1B). Grooming behavior decreased at all doses (Fig. 1C). The changes in mouse ambulatory activity caused by the association of 200 mg/kg of pulegone with 200 mg/kg of menthol were significantly lower than those predicted by the theoretical curve (Fig. 3B); however, the changes in ambulatory activity observed for the other associations did not significantly differ from the values obtained in the theoretical curves (Figs. 3A, B). Pulegone treatment decreased the grasping strength and the performance on the rotarod apparatus only at the 400 and 800 mg/kg doses (Figs. 1E, F).
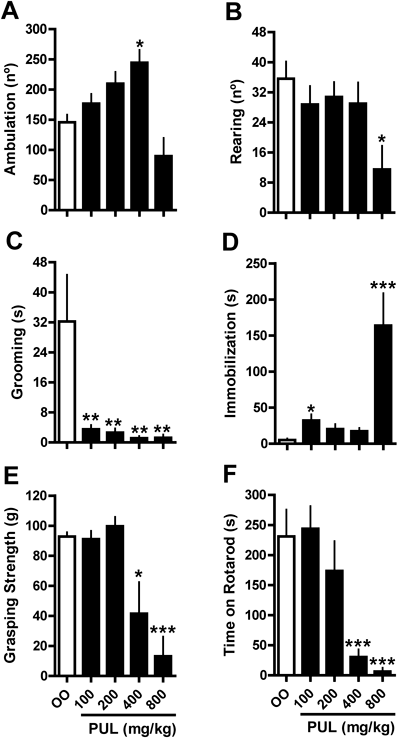
General mouse activity (ambulation, rearing, grooming and immobilization) was determined in the open field after treatment with olive oil (control; OO) or pulegone (PUL; 100–800 mg/kg, i.p.) (A–D). Immediately after evaluation in the open field, motor coordination and strength force were determined using rotarod (F) and grasping (E) test, respectively. Data are mean±S.E.M. of 8 animals. * p<0.05, ** p<0.01 and *** p<0.001 vs. control (one-way ANOVA, Newman–Keuls). The F values in A–F are, respectively: F(4,35)=8.157, F(4,35)=3.185, F(4,35)=5.785, F(4,35)=9.987, F(4,35)=10.660, F(4,35)=10.360.
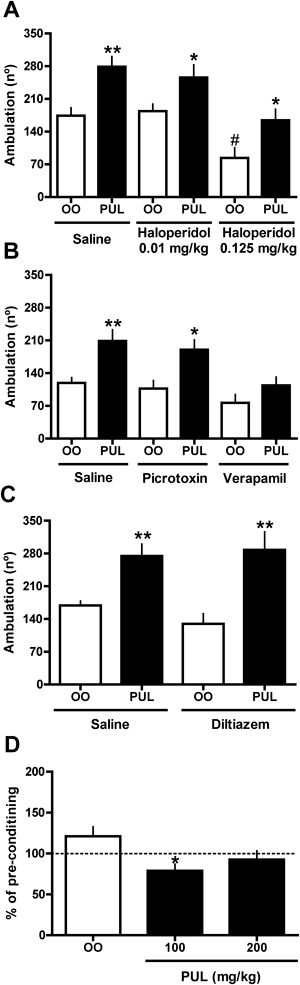
The effects of haloperidol (0.01 or 0.125 mg/kg, i.p.) (A), picrotoxin (0.25 mg/kg, i.p.) or verapamil (15 mg/kg, i.p.) (B), as well as of diltiazem (20 mg/kg, i.p.) (C) pre-treatment on the ambulation-promoting effect of pulegone (PUL; 400 mg/kg, i.p.) were determined in the open field. The motivational properties of pulegone were evaluated by pairing the drug effects on the mice with the least preferred compartment (previously determined) of a conditioned place preference apparatus (D). Data are mean±S.E.M. of 6–8 animals. * p<0.05, ** p<0.01 vs. respective control (olive oil: OO), and # p<0.05 vs. OO-saline (A and B; one-way ANOVA, Newman–Keuls). The F values in A, B and C are, respectively: F(5,37)=9.483, F(5,41)=7.864, F(3,26)=8.220. In B, * p<0.05 vs. 100% (one-sample t-test).
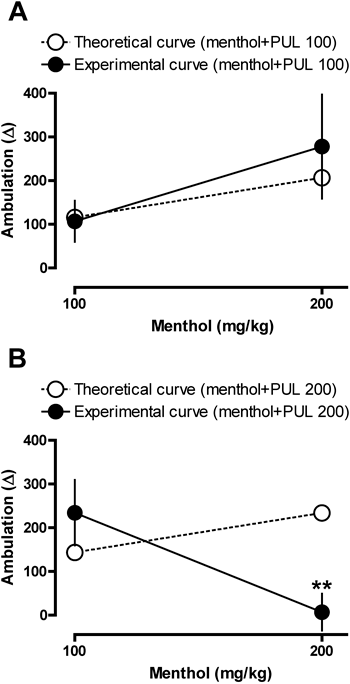
The animals were intraperitoneally treated with pulegone (100 or 200 mg/kg), menthol (100 or 200 mg/kg) or a combination of both (pulegone 100 mg/kg+menthol 100 mg/kg or pulegone 100 mg/kg+menthol 200 mg/kg, in A; pulegone 200 mg/kg+menthol 100 mg/kg or pulegone 200 mg/kg+menthol 200 mg/kg, in B). The mean amount of spontaneous locomotor activity of the group treated with olive oil (control, not shown) was used as the basal ambulation (BA); the differences between the BA and the ambulation observed in mice treated with pulegone and menthol were then calculated (ambulation observed in a specific treated group—BA=Δ effect of the specific treatment). The Δ mean of the effect produced by the administration of pulegone or menthol alone was determined (Δ mean of pulegone 100 mg/kg+Δ mean of menthol 100 mg/kg=Δ mean theoretical value of this association, Δ mean of pulegone 100 mg/kg+Δ mean of menthol 200 mg/kg=theoretical valued of this association, and so on). The effect of administering pulegone with menthol in a specific association was then compared with the theoretical value. Data are mean±S.E.M. of 8 animals or the mean of theoretical values. ** p<0.01 vs. theoretical value (one-sample t-test).
Pulegone treatment decreased the percentage of time spent in the least preferred compartment of the CPP paradigm at a dose of 100 mg/kg (Fig. 2D); however, this parameter was not significantly altered at a dose of 200 mg/kg (Fig. 2D). No significant change was observed in the control group (Fig. 2D). Pulegone treatment at 400 mg/kg increased the percentage of time spent by mice in the open arms of the EPM (Figs. 4A, B); however, flumazenil pre-treatment did not alter this effect (Fig. 4B). Diazepam increased the percentage of time spent by mice in the open arms and the percentage of entries into these arms (Fig. 4A).
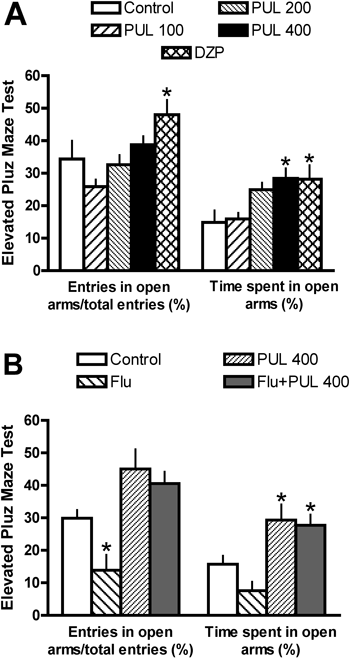
The behavioral effects induced by pulegone (PUL; 100–400 mg/kg, i.p.) or diazepam (1.0 mg/kg, i.p.; DZP) in mice observed on elevated plus maze (EPM; A). Effects of flumazenil pre-treatment (FLU; 2.5 mg/kg, i.p.) on the anxiolytic-like effect of PUL (B). Data are mean±S.E.M. of 6–8 animals. * p<0.05 vs. control (one-way ANOVA, Newman–Keuls). The F values in (A) and (B) for entries in open arms/total entries (%) are F(4,31)=4.907 and F(3,27)=10.200, respectively. The F values in (A) and (B) for time spent in open arms (%) are F(4,31)=4.672 and F(3,27)=9.436, respectively. All treatments presented in (A) increased the total number of arm entries (11.6±2.6 vs. 19.0±2.0*, 26.6±2.9*, 26.8±1.3*, 26.7±2.2*; control vs. PUL 100, PUL 200, PUL 400 and DZP 1.0 mg/kg; F(4,31)=7.715).
Pulegone has both stimulant and depressant effects on mouse behavior. In fact, pulegone induced acute psychostimulation, which was demonstrated by an increase in the ambulation of the animals at the intermediate dose administered in this study. However, the highest dose of pulegone produced only depressant effects, including significantly increased immobilization time and decreased rearing behavior. The depressant effects were also indicated by the great degree of muscular weakness and the high level of motor incoordination (ataxia) observed.
Although the pharmacological profile of pulegone shares some similarities with classical psychostimulants, such as amphetamines, Umezu16) showed that the pulegone-induced effect is distinguishable from the effects of the other psychostimulants. Like Umezu,16) we observed that pulegone induced ataxia but not stereotypy, which is a typical effect of amphetamines.29) Moreover, alterations in the general activity of animals induced by amphetamines commonly involve increases in both ambulation and rearing.30,31) However, as shown in this study, pulegone decreased rearing at high doses and did not alter rearing behavior in conditions associated with increased ambulation. Accordingly, the bell-shape of the dose–response curve for pulegone regarding the psychostimulation might reflect changes in muscular force and motor coordination; these effects could be related to central depression and/or peripheral actions, such as impairments in the transmission of neuromuscular junction. Despite the precise mechanism underlining the ataxia and muscular weakness induced by pulegone is still unknown, recently published data32) suggest that pulegone-induced reduction of action potentials amplitude in motor nerves might be involved.
Our data concerning the general activity of pulegone suggest not only that the effects of this compound are distinguishable from those of classical psychostimulants but also that the effects of pulegone strongly resemble those of classical depressants such as phenobarbital and ethanol. The maximal increase in ambulation produced by pulegone treatment was observed at a dose that also impaired muscular force and coordination. This effect is similar to that described for ethanol, which simultaneously produces behavioral stimulation and ataxia.22) In addition, it is well documented that phenobarbital either increases or decreases locomotor activity in a dose-dependent manner.33) Then, we further investigated whether picrotoxin, diltiazem or verapamil, which antagonize the psychostimulation induced by ethanol, could also antagonize pulegone-induced alterations in the locomotor activity of mice. Pulegone-induced motor excitation was insensitive to picrotoxin or diltiazem, but it was sensitive to verapamil. Although verapamil and diltiazem share the ability to block L-type calcium channels, they differ in respect to their non-specific effects, i.e., those unrelated to calcium mobilization. For instance, verapamil appears to block serotoninergic and alpha2-adrenoceptor receptors, while diltiazem do not compete for these receptors.34,35) Therefore, our data suggest that GABAA receptor and voltage-dependent L-type calcium channel activation are not involved in the psychostimulation induced by pulegone, in contrast to previous results observed following ethanol treatment.22,23) These experiments evidence that pulegone-induced behavioral alterations are also pharmacologically distinguishable from those induced by classical depressants, despite the similarities among the observed behavioral alterations. The data also suggest that some molecular targets for verapamil (others than calcium channels) should be involved in pulegone-induced motor excitation, a possibility that requires future investigations. Moreover, our results highlight the potential use of verapamil in selected patients (those without cardiac depression)6,36) under intoxication of pulegone-containing natural products aimed to inhibit the psychostimulation.
We evaluated the sensitivity of pulegone-induced acute psychostimulation to the dopamine D2 receptor antagonist and observed no significant impairment of the ability of pulegone to increase ambulation in mice pre-treated with haloperidol. It is important to mention that we used doses of haloperidol that have been demonstrated to antagonize amphetamine- and pulegone-induced psychostimulation.16,19) The effects of dopamine on motor activity have been extensively investigated and a body of evidence suggests that locomotor activity is mainly controlled by D1, D2, and D3 dopamine receptors.37) The activation of D1 receptors, that appear to be exclusively expressed on the postsynaptic neurons, has a stimulatory effect on locomotor activity; however, the activation of D2 receptors elicits a more complex pattern of effects, since they result from both presynaptic and postsynaptic interactions.37) Activation of presynaptic D2 autoreceptors causes a decrease in dopamine release that results in decreased locomotor activity, while activation of postsynaptic receptors stimulates locomotion; thus, drugs that directly or indirectly stimulate D2 receptors can induce a biphasic effect, leading to decreased activity at low doses and behavioral activation at high doses.37) On the other hand, the D3 receptor seems to play an inhibitory influence on locomotion, which appears to involve both presynaptic and postsynaptic receptors.37) Accordingly, despite our data do not preclude the involvement of dopamine in the pulegone-induced increase in mouse locomotor activity through D1 receptor activation, as suggested by Umezu,16) and/or through D3 receptor blockage, our study does not support the involvement of D2 receptor in the pulegone-induced motor excitation, which is contrary to previously reported results.16)
Studying the motivational properties of pulegone, we observed that the lowest dose induced CPA instead of CPP, suggesting that this terpenic compound has aversive properties. The dose of pulegone related to CPA induction also altered the immobilization of the animals in the open field test. In this case, the observed immobilization cannot be explained by the alteration in muscular force or motor incoordination because the dose required did not alter the grasping test or rotarod performance. Therefore, taking into account that immobilization is considered a measure of emotionality,38) the incresed immobilization time observed in the open field test also suggests that the lowest dose of pulegone had aversive properties. Interestingly, it has been suggested that the more animals are inhibited in the open field, the less they will groom39); indeed, rodents show low levels of grooming activity when exposed to aversive environments such as sessions in a high illuminated open field.40) Therefore, our data suggest that the effects of low doses of pulegone on grooming behavior could be related to its aversive properties, while the decreased grooming observed with high doses may be due to alterations in motor coordination and force.
Some essential oils that are used in aromatherapy appear to improve the subjective sense of well-being,41) and our data strongly suggest that pulegone is not involved in this alteration. Moreover, judging only by the pulegone content of these natural products, our data indicate no abuse potential for these products, which is an important matter concerning public health and governmental decisions related to the ban or restriction of the sale of such products.
It has been reported that peppermint oil increases alertness in humans.9) Although pulegone-induced psychostimulation cannot be disregarded when considering this effect, the high and toxic doses of this compound that are necessary to alter mouse locomotor activity strongly suggest that pulegone is not the main component in those natural products that increases alertness. However, it is possible that a pharmacological interaction, such as synergism or an additive effect, could occur between pulegone and other substances that are present in essential oils. This suggestion is based on the fact that many essential oils are complex mixtures with a large amount of menthol and menthone,4) which also have psychostimulant effects.42,43) To test this hypothesis, we investigated whether some type of pharmacological interaction could occur between pulegone and menthol. Our data suggest the presence of additive and synergic interactions between these two natural products; no difference was observed between the theoretical and experimental curves of the addictive effects when pulegone was administered at a low dose (100 mg/kg), suggesting that the pulegone and menthol effects are additive in this condition. Moreover, even though the effect of the association of intermediate doses of both pulegone (200 mg/kg) and menthol (200 mg/kg) could be interpreted as an antagonistic interaction (because the experimental value was below the theoretical one), the results seem to reflect a synergic effect. This interpretation is based on the observation that pulegone did not significantly alter motor activity at the highest dose; therefore, minimal or no changes in mouse ambulatory activity would be expected if synergic interactions occurred between the intermediates doses. Hence, some of the effects of natural products that contain pulegone and menthol could be explained by a phytocomplex formed by these two terpenic compounds.
Natural products that contain a large amount of pulegone have been used as sedatives.1) Therefore, we analyzed the effects of pulegone treatment on the performance of mice in the EPM, a well-accepted paradigm to test the anxiolytic/anxiogenic properties of drugs.44–46) Like diazepam, pulegone appears to have anxiolytic-like activity, as suggested by the observed increase in time spent in the open arms, which is considered to be a reliable index of anxiolytic activity.46,47) Moreover, pulegone-induced EPM behavioral alteration was not altered by flumazenil pre-treatment, suggesting that the anxiolytic-like effects of pulegone do not involve the benzodiazepine site of the GABAA receptor. The high and toxic dose of pulegone that was necessary to produce an anxiolytic-like effect in mice suggests that this compound is not the only substance involved in the anxiety reduction that has been reported following the use of pulegone-containing essential oils by humans. Thus, pharmacological interactions between pulegone and other constituents in essential oil should also be considered and further investigated in this respect.
Finally, it is important to note that our data concerning the motivational properties of pulegone do not disagree with the observation that pulegone has anxiolytic-like effects. It has been reported that phenobarbital may induce either anxiolytic-like effects in the EPM or CPA.48) Furthermore, the possible dissociation between the rewarding and anxiolytic properties of drugs has also been suggested by other drug studies.49) Therefore, drugs that reduce anxiety do not necessarily have secondary positive reinforcing properties.
In conclusion, pulegone has a dose-dependent dual effect on mouse behavior, which acts as either a stimulant or a depressant. Pulegone induces a verapamil-sensitive psychostimulant effect that appears to independ on the opening of L-type calcium channels. Moreover, pulegone possesses negative reinforcing properties and has anxiolytic-like effects that are unrelated to the benzodiazepine site of the GABAA receptor and might be a part of a phytocomplex that is formed in natural products and acts in an addictive or synergic way with other monoterpenic compounds. Therefore, our data provide some explanation of the pharmacological and toxicological actions ascribed to pulegone-containing essential oils and highlight the use of verapamil in selected cases of intoxication with this natural compound.
The authors are indebted to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and to the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for the undergraduate research program scholarship. We are also indebted to the Pró-Reitoria de Pós-Graduação e Pesquisa da Universidade Federal de Uberlândia (PROPP-UFU) for financial support.