2014 Volume 37 Issue 6 Pages 884-891
2014 Volume 37 Issue 6 Pages 884-891
Potent ligands of peroxisome proliferator-activated receptor γ (PPARγ) such as thiazolidinediones (pioglitazone, troglitazone, etc.) improve insulin sensitivity by increasing the levels of adiponectin, an important adipocytokine associated with insulin sensitivity in adipose tissue. Several constituents from medicinal plants were recently reported to show PPARγ agonist-like activity in 3T3-L1 cells, but did not show agonistic activity at the receptor site different from thiazolidinediones. Our recent studies on PPARγ agonist-like constituents, such as hydrangenol and hydrangeic acid from the processed leaves of Hydrangea macrophylla var. thunbergii, piperlonguminine and retrofractamide A from the fruit of Piper chaba, and tetramethylkaempferol and pentamethylquercetin from the rhizomes of Kaempferia parviflora, are reviewed.
In modern society, the number of patients with diabetes mellitus and its preliminary groups are increasing worldwide, and diabetes mellitus is a chronic and often undiagnosed or inadequately treated disease. Type 2 diabetes is closely associated with other metabolic disorders, such as hypertension, cardiovascular disease, and atherosclerosis. Insulin resistance is an important marker for developing type 2 diabetes. The roles of life-style change and weight loss in preventing diabetes have been proven in clinical trials. Several oral hypoglycemic agents and the anti-obese drug orlistat have been shown to significantly decrease progression to diabetes. However, more effective and safe medicines are required.1)
Thiazolidinedione-type compounds such as pioglitazone and rosiglitazone are potent insulin sensitizers currently used clinically to treat type 2 diabetes. Thiazolidinedione-type compounds were originally identified based on their antihyperglycemic activity, but they are also able to improve other abnormalities associated with type 2 diabetes, such as hyperlipidemia, atherosclerosis, hypertension, and chronic inflammation. Thiazolidinedione-type compounds are potent ligands of peroxisome proliferator-activated receptor γ (PPARγ) and improve insulin sensitivity by increasing the levels of adiponectin, an important adipocytokine associated with insulin sensitivity in adipose tissue, and by decreasing free fatty acid and tumor necrosis factor α (TNF-α) levels in diabetic subjects and animal models (in vivo) and in adipocytes (in vitro).2–5) It is well established that PPARγ agonists such as thiazolidinedione-type compounds promote the adipogenesis of a pre-adipocyte cell line (3T3-L1), and so the cells have been used for the development of anti-diabetic compounds.6–8)
Previous studies using a reporter gene assay for PPARα/γ successfully demonstrated their various agonists and antagonists from natural resources.9–13) In the course of our studies on searching for anti-diabetic compounds, we examined the effects of various extracts of medicinal plants and isolated compounds on enhancement of triglyceride (TG) accumulation, as a marker of adipogenesis, in 3T3-L1 cells. Among the compounds tested, several constituents exhibited adipogenic effects similar to PPARγ agonists in 3T3-L1 cells, although they did not act as the agonist for the receptor levels using a nuclear cofactor assay system. In this paper, we review our recent studies on the PPARγ agonist-like compounds having less agonistic activity for the receptor.14–19)
The Saxifragaceae plant, Hydrangea (H.) macrophylla Seringe var. thunbergii Makino, is native to Japan. The processed leaves of this plant (sweet hydrangea leaf) are listed in the Japanese Pharmacopoeia XVI and are currently used as an oral refrigerant and as a sweetener for diabetic patients. Previously, we reported the isolation and structural determination of many chemical constituents with anti-allergic and anti-bacterial activities from the processed leaves and the dried leaves of this plant.20–25)
In the course of searching for anti-diabetic constituents from natural medicines, we found that the ethyl acetate fraction of the processed leaves of H. macrophylla var. thunbergii promoted the accumulation of triglyceride in 3T3-L1 cells. Anti-microbial and anti-allergic effects of the constituents of this herb have been reported before; however, anti-diabetic effects have not. Isolated constituents [hydrangenol (1), phyllodulcin (3), thunberginols A–F (4, 6, 8–10), and hydrangeic acid (2)] from the processed leaves and hydrangenol 8-O-glucoside (5) and phyllodulcin 8-O-glucoside (7) from the un-processed leaves were examined for effects on adipogenesis in 3T3-L1 cells. As shown in Fig. 1, 3-phenyldihydroisocoumarins [hydrangenol (1) and phyllodulcin (3)] and a stilbene [hydrangeic acid (2)] promoted the accumulation of TG in the cells. Compounds 8–10 reduced TG levels at a high concentration (100 µM), suggesting cytotoxic effects at high concentrations. With regard to the structural requirements for the activity, a hydroxy group at the 6-position and a glucopyranosyl group reduced the activity.
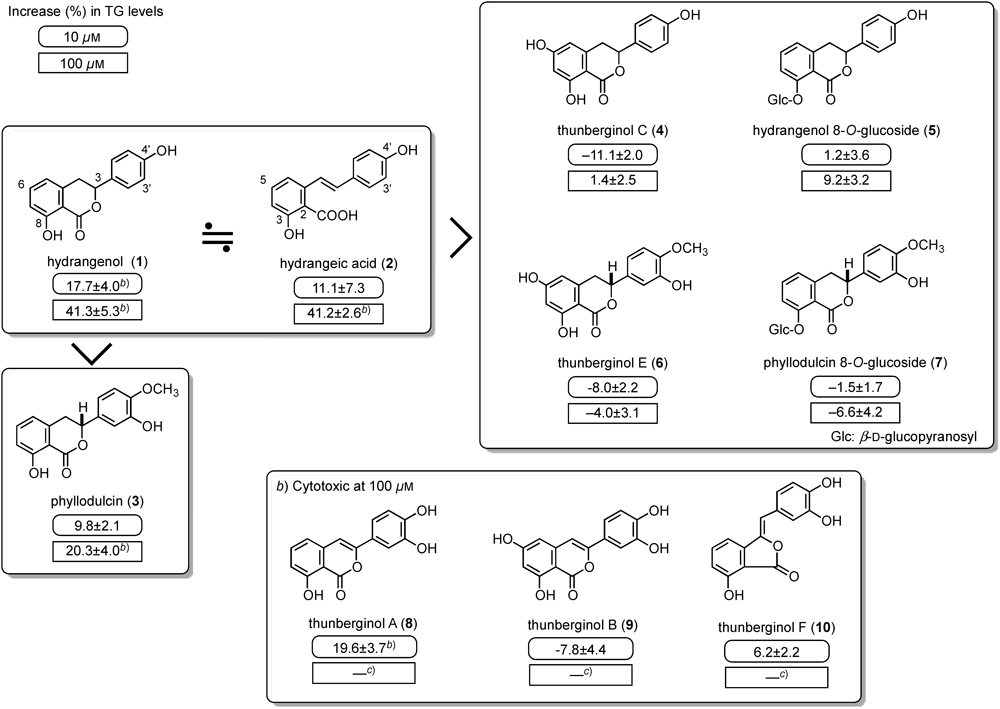
Murine 3T3-L1 cells [Health Science Research Resources Bank (Osaka, Japan)] (5.0×104 cells/well) in DMEM supplemented with 10% FBS were seeded into a 48-well multiplate. After 24 h, the differentiation was induced by changing the medium to a differentiation medium [DMEM (high glucose) supplemented with 10% FBS, 1 µM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, 5 µg/mL insulin]. After 3 d, the differentiation medium was replaced with a maintenance medium [DMEM (high glucose) supplemented with 10% FBS and 5 µg/mL insulin]. After 4 d (on day 8), the medium was removed and H2O (200 µL/well) was added to each well, and then the cells were sonicated. The triglyceride (TG) level in the sonicate was determined. Test compound dissolved in DMSO was added to the differentiation and maintenance media (final DMSO conc. was 0.1%). Values represent means±S.E.M. of % increase in TG levels (n=4). a) Data taken from ref. 14. Significantly different from the control group, b) p<0.01. c) Cytotoxic effect was observed at 100 µM.
Furthermore, compounds 1 and 2 (3–100 µM) concentration-dependently enhanced the release of adiponectin into the medium and the uptake of 3H-2-deoxyglucose (2-DG) in 3T3-L1 cells. As shown in Table 1, the gene expression of adiponectin, glucose transporter 4 (GLUT4), fatty acid-binding protein (aP2), and PPARγ2 were increased by treatment with 2 similar to a PPARγ agonist, troglitazone.26) Hydrangenol (1) also showed a similar effect on gene expression.
| Ratio (target gene/β-actin mRNA) | ||||
|---|---|---|---|---|
| Hydrangeic acid (2) | ||||
| Conc. (μM) | 0 | 30 | 100 | |
| Adiponectin | 1.00±0.11 | 1.00±0.06 | 1.57±0.04b) | |
| GLUT4 | 1.00±0.12 | 1.11±0.05 | 1.80±0.12c) | |
| aP2 | 1.00±0.03 | 1.49±0.16b) | 2.19±0.14c) | |
| TNF-α | 1.00±0.16 | 0.40±0.02c) | 0.39±0.07c) | |
| PPARγ2 | 1.00±0.06 | 1.16±0.08 | 1.89±0.14c) | |
| Ratio (target gene/β-actin mRNA) | ||||
| Retrofractamide A (15) | ||||
| Conc. (μM) | 0 | 3 | 10 | 30 |
| Adiponectin | 1.00±0.15 | 2.01±0.04c) | 2.51±0.22c) | 2.39±0.05c) |
| GLUT4 | 1.00±0.15 | 2.00±0.20c) | 2.10±0.17c) | 3.45±0.30c) |
| IRS-1 | 1.00±0.13 | 1.10±0.04 | 1.27±0.18 | 1.49±0.03b) |
| PPARγ2 | 1.00±0.12 | 1.16±0.06 | 1.16±0.07 | 1.66±0.15c) |
| Ratio (target gene/β-actin mRNA) | ||||
| Pentamethylquercetin (25) | ||||
| Conc. (μM) | 0 | 3 | 10 | 30 |
| Adiponectin | 1.00±0.04 | 1.67±0.14c) | 1.91±0.07c) | 2.59±0.07c) |
| GLUT4 | 1.00±0.09 | 2.01±0.07c) | 2.25±0.08c) | 3.62±0.21c) |
| aP2 | 1.00±0.11 | 1.34±0.03 | 1.59±0.05c) | 2.10±0.14c) |
| PPARγ2 | 1.00±0.05 | 1.84±0.06c) | 1.99±0.07c) | 2.42±0.18c) |
| C/EBPα | 1.00±0.08 | 1.79±0.09c) | 1.50±0.10c) | 1.38±0.02b) |
| C/EBPβ | 1.00±0.11 | 1.73±0.11c) | 3.01±0.08c) | 3.82±0.23c) |
3T3-L1 cells (1.0×106 cells/well) in DMEM supplemented with 10% FBS were seeded into a 6-well multiplate. After the differentiation, the medium was replaced with the maintenance medium. On day 8, total RNA was extracted and reverse transcribed to cDNA. Then a real-time PCR was carried out. The abundance of each gene product was calculated by relative quantification, with values for the target genes normalized to β-actin mRNA. The test compound dissoved in DMSO was added to the differentiation and maintenance media. Values represent the mean±S.E.M. (n=3). a) Data taken from refs. 15, 16, and 19. Significantly different from the control group, b) p<0.05, c) p<0.01.
Next, PPARγ agonistic activity was examined using a nuclear receptor cofactor assay system (EnBio RCAS for PPARγ, EnBioTec Laboratories). This system is a cell-free assay system using nuclear receptors and cofactors (coactivator, corepressor) to screen chemicals. As a result, 2 (1–100 µM) showed weak activity apparently different from troglitazone (Fig. 2).
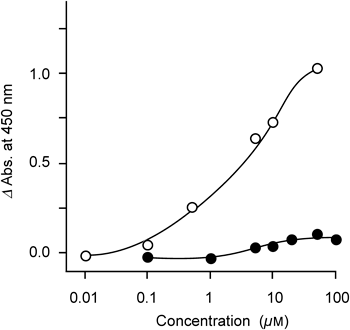
●: hydrangeic acid (2), ○: troglitazone. Agonistic activity toward PPARγ was examined using a nuclear receptor cofactor assay system (EnBio RCAS for PPARγ). The experiment was done in duplicate. a) Data taken from ref. 15.
As an in vivo experiment, effects of hydrangenol (1) and hydrangeic acid (2) on blood glucose, TG, and free fatty acid (FFA) levels in diabetic KK-Ay mice, as a model of type 2 diabetes, were examined. Compounds 1 and 2 significantly lowered blood glucose levels 2 weeks after administration at 200 mg/kg/d in diabetic KK-Ay mice (Table 2). In addition, 1 and 2 at a dose of 200 mg/kg/d decreased plasma free fatty acids (FFA) levels, which is believed to be one of the factors inducing insulin resistance, similar to troglitazone.
| Dose (mg/kg, p.o.) | n | Blood glucose levels (mg/dL) | |||
|---|---|---|---|---|---|
| 0 w | 1 w | 2 w | |||
| Normal (C57BL/6) | — | 6 | 239.1±11.2c) | 253.2±12.8c) | 236.1±9.3c) |
| Control (vehicle) | — | 14 | 343.9±24.7 | 482.1±10.7 | 491.3±8.9 |
| Hydrangenol (1) | 100 | 13 | 336.3±20.0 | 389.1±23.0c) | 422.7±15.5b) |
| 200 | 13 | 336.0±20.6 | 397.5±26.9b) | 411.2±25.4c) | |
| Control (vehicle) | — | 6 | 339.6±19.9 | 381.9±32.9 | 387.9±42.3 |
| Hydrangeic acid (2) | 100 | 6 | 330.4±14.9 | 393.3±40.3 | 400.6±38.1 |
| 200 | 6 | 326.2±25.3 | 334.7±12.7 | 332.0±10.9c) | |
| Control (vehicle) | — | 7 | 324.9±24.6 | 355.1±41.2 | 470.3±19.5 |
| Troglitazone | 50 | 7 | 322.1±28.7 | 361.9±28.0 | 439.3±16.6 |
| 100 | 7 | 323.3±19.2 | 315.6±21.4 | 338.2±30.3c) | |
Test samples suspended in 5% acacia solution and vehicle (5% acacia solution) were given orally to male KK-Ay mice (5 weeks old) once a day for 2 weeks. Plasma glucose levels of non-fasted KK-Ay mice were determined using commercial kits (Glucose CII-test Wako). C57BL/6 mice were used as non-diabetes mice (normal mice). Values represent the means±S.E.M. a) Data taken from ref. 15. Significantly different from the control group, b) p<0.05, c) p<0.01.
Recently, PPAR dual agonists were developed to combine the triglyceride-lowering and high-density lipoprotein cholesterol-elevating effects of PPARα agonists (e.g. fibrates) with the insulin sensitivity-improving effects of PPARγ agonists (e.g. thiazolidiones).27) In our experiments, reductions in the weight of epididymal fat, mesenteric fat, and nephritic fat were observed by treatment with 2. These results indicate the possibility of PPARα agonist-like effects by 2, although PPARα agonistic activity of 2 (100 µM) was not observed using the nuclear receptor cofactor assay system (EnBio RCAS for PPARα).
2.2. Retrofractamide A and Related Compounds from the Fruit of Piper chaba16–18)Piper chaba Hunter (syn. P. retrofractum Vahl., Piperaceae) is widely distributed in Southeast Asia. In Thailand, the fruit of this plant is called “Dee Plee” and has a wide range of applications in traditional medicine, for example, as an antiflatulent, expectorant, antitussive, antifungal, uterus-contracting agent, sedative-hypnotic, appetizer, and counterirritant.28) We have been conducting studies to characterize this natural medicine and previously reported that 47 constituents could be isolated from the 80% aqueous acetone extract of the fruit of P. chaba.29–31) The aqueous acetone extract and the isolates were found to show a range of protective effects: they decreased the rate of ethanol- or indomethacin-induced gastric lesions in rats; they inhibited an increase in serum aspartate aminotransaminase (sAST) and serum alanine aminotransaminase (sALT) levels induced by D-galactosamine (D-GalN)/lipopolysaccharide (LPS)-induced liver injury in mice; and they reduced the rate of cell death in primary cultured mouse hepatocytes induced by D-GalN/ TNF-α.30–32)
Recently, spice-derived compounds (e.g. allyl isothiocyanate, zingerone, and curcumin) were reported to markedly inhibit the cellular production of proinflammatory mediators such as TNF-α and nitric oxide, and significantly inhibited the release of monocyte chemoattractant protein-1 (MCP-1) from 3T3-L1 adipocytes.33) Furthermore, capsaicin, a well known transient receptor potential vanilloid type-1 (TRPV1) agonist, was reported to prevent adipogenesis in stimulated 3T3-L1 cells,33,34) but it induced the up-regulation of adiponectin.35) On the other hand, the effects of piperine (11), which was reported as a TRPV1 agonist,36) and related amide constituents on 3T3-L1 cells have not been examined.
Among the amide constituents, piperlonguminine (14), retrofractamides A (15), B (16), and C, and bracyhstamide B significantly enhanced the accumulation of TG levels at 1–30 µM (Fig. 3). However, piperine (11) at 1–30 µM did not show a significant inhibition of TG accumulation in our experimental conditions, although capsaicin was reported to reduce the adipogenesis of 3T3-L1 cells.33,34)
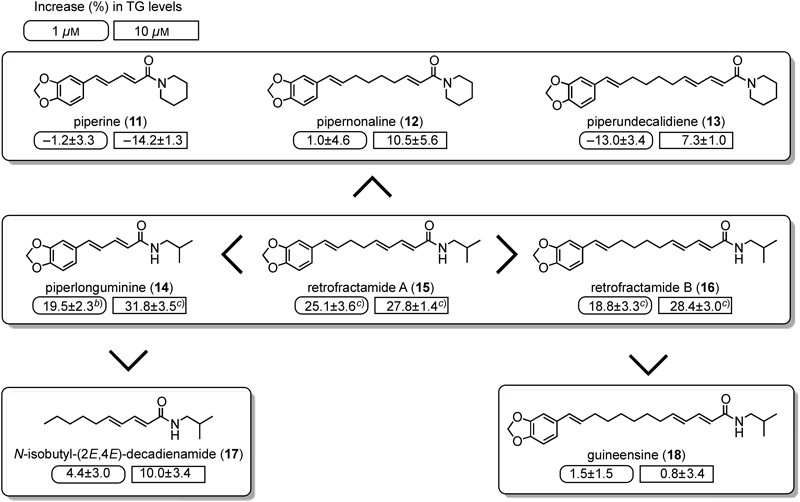
The experiment was performed as described in Fig. 1. Values represent the means±S.E.M. (n=4). a) Data taken from ref. 16. Significantly different from the control group, b) p<0.05, c) p<0.01.
With regard to the structural requirements of the amide constituents for the activity, all amide structures of 14–16 are composed of an isobutyl amine moiety, but 11–13 composed of a piperidine ring showed less effect. These findings suggested that the amide structure composed of an isobutyl amine moiety was important. Furthermore, the amide constituent (17) lacking an aromatic ring had weak activity, and the length of the carbon chain between the aromatic ring and amide moiety and a double bond conjugated to an α,β-unsaturated carbonyl group were also suggested to be important.
Compounds 14 and 15 concentration-dependently increased the uptake of 2-DG and the release of adiponectin into the medium like troglitazone. The expression of adiponection and GLUT4 mRNA was significantly increased by 15. In addition, 15 significantly increased IRS-1 and PPARγ2 mRNA levels at 30 µM (Table 1). Piperlonguminine (14) also showed similar effects on the gene expression. Furthermore, several derivatives of the amide group in 15 were synthesized, and their adipogenic effects in 3T3-L1 cells were examined. Among the compounds tested, amides composed of an n-butyl or n-pentyl amine showed stronger activity. The amide with the n-pentyl amine moiety significantly increased the uptake of 2-DG into the cells, and also increased the mRNA levels of adiponectin, GLUT4, aP2, and PPARγ2, in a similar manner as the PPARγ agonist troglitazone, although this synthetic compound as well as 14 and 15 had less agonistic activity against PPARγ using the nuclear receptor cofactor assay system (EnBio RCAS for PPARγ).
The effects of 14 and 15 on diabetic animals are not yet clarified, but the plasma levels of piperlonguminine (14) and retrofractamide A (15) after oral administration in mice were compared with those of piperine (11) to obtain information on doses for in vivo study. Analyses of plasma from mice treated with 11, 14, and 15, and an extract of the fruit showed that concentrations of 14 were higher than those of 11 and 15 (Table 3). The effective concentrations of 14 and 15 for PPARγ agonist-like effects in 3T3-L1 cells were 1–30 µM, and the respective Cmax at 20 mg/kg of these compounds were 1.97 µg/mL (7.2 µM) and 0.43 µg/mL (1.3 µM). With regard to effective doses in vivo, an administration regimen of several times per day of more than 20 mg/kg would be necessary in light of their Cmax and half-lives.
| Dose (mg/kg, p.o.) | Tmax (h) | Cmax (mg/mL) | AUC0–24 h (mg·h/mL) | |
|---|---|---|---|---|
| 80% Acetone Ext. | 50b) | 1.0 | 0.402 | 2.220 |
| (20.7% piperine) | 100c) | 1.2 | 0.928 | 6.160 |
| Piperine (11) | 5 | 0.8 | 0.192 | 0.527 |
| 10 | 0.8 | 0.377 | 1.620 | |
| 20 | 1.0 | 0.770 | 3.310 | |
| Piperlonguminine (14) | 5 | 0.9 | 0.316 | 0.914 |
| 10 | 1.2 | 0.708 | 2.510 | |
| 20 | 1.0 | 1.970 | 9.060 | |
| Retrofractamide A (15) | 5 | 1.1 | 0.090 | 0.327 |
| 10 | 0.9 | 0.191 | 0.765 | |
| 20 | 1.1 | 0.425 | 1.710 |
a) Data taken from ref. 17. b, c) These doses were equivalent to ca. 10 and 20 mg/kg of piperine (11). Values represent the means of 6 mice.
Piperine (11) was reported to inhibit CYP3A4 and P-glycoprotein, which are expressed in enterocytes and determine the bioavailability of many drugs,37–39) and also to enhance the plasma concentrations of several drugs and polyphenols in foods.40–44) In our study, the “area-under-the-curve” (AUC) of 11 increased following in vivo administration of the extract, suggesting that the other constituents in the extract inhibit the metabolism of 11.
2.3. Methoxyflavonols from the Rhizome of Kaempferia parviflora19)Previous studies have demonstrated that flavonoids and/or flavonoid-rich fractions ameliorate experimental diabetes in mice and rats.45–47) We also reported inhibitory activities of various flavonoids against aldose reductase, a key enzyme in the polyol pathway, and the formation of advanced glycation end-products (AGEs) in vitro, and clarified several structural requirements for the activity.48,49) However, several flavonoids were reported to inhibit insulin signals in adipocytes.50,51)
The effects of 44 flavonoids on the adipogenesis of 3T3-L1 cells in order to find PPARγ agonist-like compounds were examined. Among the compounds tested, tetramethylkaempferol (24), pentamethylquercetin (25), and 3,7,4′-trimethylkaempferol (26) from the rhizomes of Kaempferia parviflora Wall. ex Baker52) promoted the accumulation of TG in a concentration-dependent manner at 1–30 µM (Fig. 4).
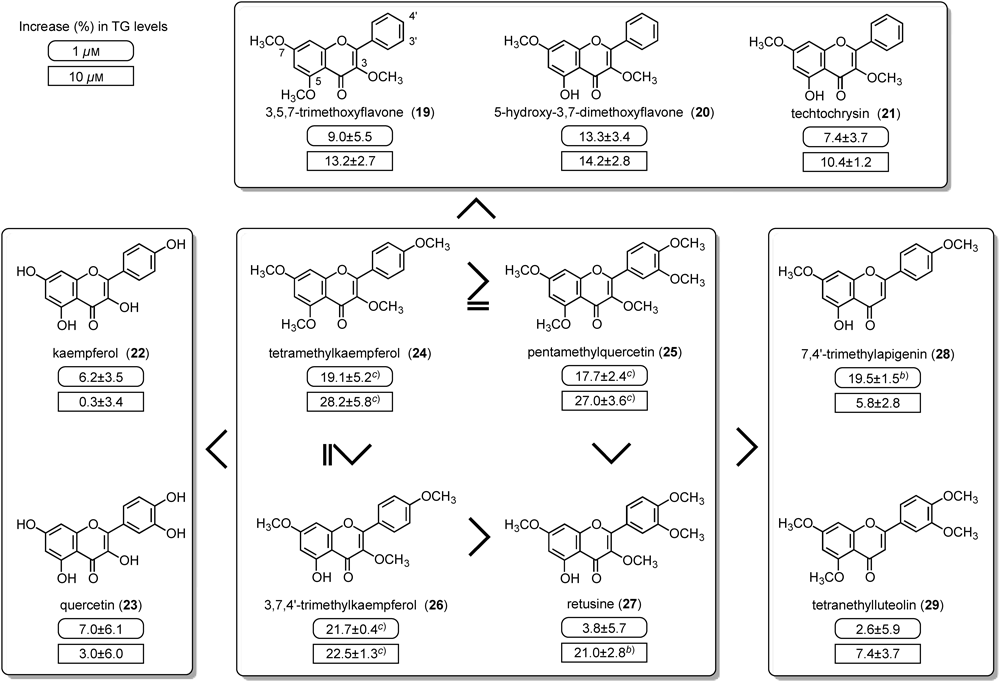
The experiment was performed as described in Fig. 1. Values represent the means±S.E.M. (n=4). a) Data taken from ref. 19. Significantly different from the control group, b) p<0.05, c) p<0.01.
Cytotoxic effects of flavonoids have been reported in many cell lines.53–55) The marked reduction in TG levels observed on treatment with several flavonoids (e.g. luteolin, baicalein, 5,7-dimethoxyflavone, tetramethylluteolin, butein) at higher concentrations (30 and/or 100 µM) may depend on cytotoxic effects, although further mechanisms such as the inhibition of insulin signal transduction or cell adhesion mechanisms should be examined.
With regard to the structural requirements of flavonoids for the activity, it was found that: (1) most flavonoids having hydroxy groups lacked the effect; (2) flavonols with methoxy groups showed stronger effects particularly those with a methoxy group at the 3-position; and (3) a methoxy group of flavonols at the B ring was also important.
Tetramethylkaempferol (24), pentamethylquercetin (25), and 3,7,4′-trimethylkaempferol (26), significantly increased the amount of adiponectin released into the medium as well as the uptake of 2-DG into the cells. Furthermore, 24 and 25 also increased mRNA levels of adiponectin, GLUT4, and aP2.
Previously, Nomura et al. reported that flavonoids [e.g. apigenin, luteolin, fisetin, kaempferol (22), quercetin (23), and genistein] inhibited insulin signal transduction, and inhibited the translocation of GLUT4 and uptake of glucose in MC3T3-G2/PA6 adipose cells.51) In our study, consistent with the previous study,51) kaempferol (22) concentration-dependently inhibited the uptake of 2-DG under conditions in which the compounds were incubated with insulin 20 min before the incubation with 2-DG in 3T3-L1 adipocytes (on day 8).
The CCAAT/enhancer-binding protein (C/EBP) family plays an important role in growth and differentiation in many tissues and cell types. Studies in adipogenic cell lines have shown that hormonal induction of differentiation is rapidly followed by the expression of C/EBPβ and δ. Levels of these proteins peak within several days and then begin to drift downward, coinciding with a rise in C/EBPα and PPARγ. C/EBPα and PPARγ induce changes in gene expression characteristic of mature adipocytes. Although the relation between C/EBPα and PPARγ is complicated, there is a cross-regulation between C/EBPα and PPARγ during adipogenesis and the major role of C/EBPα is centered on maintaining the expression of PPARγ and promoting full insulin sensitivity.56–58)
Pentamethylquercetin (25) also increased the mRNA levels of PPARγ2 and C/EBPα and β as well as adiponectin, GLUT4, and aP2 (Table 1), although, in contrast to troglitazone, they did not activate PPARγ directly in the nuclear receptor cofactor assay. In addition, tetramethylkaempferol (24) showed similar effects. Our findings suggest that the mechanism of action by 24 and 25 is different from that by PPARγ agonists, and an enhancement of C/EBPs expression is involved in the PPARγ agonist-like effects of 24 and 25. Chen et al. also reported that pentamethylquercetin (25) up-regulated adiponectin expression in differentiated 3T3-L1 cells via a mechanism that implicated PPARγ together with TNF-α and IL-6.59) With regard to in vivo study, several attractive studies about anti-diabetic and anti-obese effects of 25 in several animal models were reported.60–63)
The detailed mechanisms of 24 and 25 are still unclear, because many transcriptional factors are involved in agipogenesis. For example, early adipogenic transcription factors [e.g. cAMP response element binding protein (CREB), Krüppel-like factor (KLF) 4, KLF5, glucocorticoid receptor (GR), C/EBPβ and δ, signal transducer and activator of transcription (STAT) 5A/B] are activated directly by the adipogenic hormone cocktail consisting of glucocorticoids, insulin, a cAMP-elevating agent, and fetal bovine serum (FBS). This first set of adipogenic factors directly induces subsequent adipogenic factors [e.g. sterol regulatory element-binding protein (SREBP)-1c, thyroid hormone receptor (TR), C/EBPα, PPARγ, liver X receptor (LXR), KLF15], which coordinate to induce genes linked to the adipocyte phenotype.64)
3-Phenyldihydroisocoumarins [hydrangenol (1), etc.] and a stilbene [hydrangeic acid (2)] from the processed leaves of H. macrophylla var. thunbergii, several amide constituents [piperlonguminine (14), retrofractamide A (15), etc.] isolated from the fruit of P. chaba, and methoxyflavonols [tetramethylkaempferol (24), pentamethylquercetin (25), etc.] promoted adipogenesis of 3T3-L1 cells. These compounds significantly increased the amount of adiponectin released into the medium as well as the uptake of 2-DG into the cells. They also increased mRNA levels of adiponectin, GLUT4, etc. like troglitazone, but unlike troglitazone, they had less agonistic activity for PPARγ. These findings suggest that these compounds (1, 2, 14, 15, 24, and 25) are promising seed compounds for new type of anti-diabetic agents, and their molecular targets need to be clarified.
This research was supported in part by the Academic Frontier Project, and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.