2014 Volume 37 Issue 7 Pages 1132-1138
2014 Volume 37 Issue 7 Pages 1132-1138
St. John’s wort (SJW), or Hypericum perforatum, is a perennial herb that has been used in the treatment of depression in several countries. Though its therapeutic effect on depression has been extensively studied, its influence on metabolic syndrome is yet to be fully characterized. Therefore, we investigated the effect of SJW extract on adipocyte differentiation and its anti-inflammatory effects by using 3T3-L1 preadipocytes. Oil Red O staining indicated that SJW promotes adipocyte differentiation, while immunoblots indicated that SJW increases the expression of peroxisome proliferator activated receptor γ (PPARγ), a nuclear receptor regulating adipocyte differentiation, and adiponectin, an anti-inflammatory adipokine. Furthermore, the anti-inflammatory activity of SJW was demonstrated by its inhibition of the activation of nuclear factor-κB (NF-κB), an inflammatory transcription factor. Stimulation of mature 3T3-L1 adipocytes by tumor necrosis factor-α (TNF-α) decreased the expression of the NF-κB inhibitor IκBα, and increased its phosphorylation. Treatment with SJW further decreased the TNF-α-induced perturbation in IκBα expression and phosphorylation, which indicated that SJW mediated the inhibition of NF-κB activation. In addition, SJW decreased the TNF-α-induced increase in the mRNA levels of pro-inflammatory adipokines, interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1). Collectively, our results indicate that SJW treatment could promote adipocyte differentiation probably through its anti-inflammatory activity, which in turn suggests that SJW has the potential to minimize the risk factors of metabolic syndrome.
Obesity and its related metabolic syndrome predisposes individuals to critical disorders, such as insulin resistance, diabetes, and cardiovascular disease.1,2) The increased volume of the adipose tissue in obesity reflects an increase in the number, size, or both of the adipocytes.3) The increase in the size of the adipocytes could be attributed to their excessive lipid storage. The large adipocytes in obesity secrete pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), or monocyte chemoattractant protein-1 (MCP-1), and induce glucose and lipid metabolism disorders.4–6) IL-6 and MCP-1 are regulated by nuclear factor-κB (NF-κB), one of the most critical inflammatory transcription factors, which leads to an increase of pro-inflammatory cytokines in adipose tissue.7,8) The activity of NF-κB is regulated by endogenous κB inhibitors (IκB), which form complexes with NF-κB to keep it in an inactive state in the cytoplasm.9) Phosphorylated IκBα (P-IκBα) is degraded by the ubiquitin proteasome system, which results in the release and nuclear translocation of NF-κB, and allows NF-κB to regulate gene expression.10,11)
On the other hand, normal adipocytes secrete anti-inflammatory proteins, such as adiponectin, that regulate glucose and lipid metabolism.12–14) The expression of adiponectin is regulated by peroxisome proliferator-activated receptor (PPAR) γ, which is a member of the PPAR subfamily of nuclear hormone receptors.3,15) PPARγ binds to a PPAR-responsive element in the promoter of adiponectin, and increases adiponectin gene transcription.16) The ligand of PPARγ is used to attenuate insulin resistance because activation of PPARγ increases adiponectin secretion from small adipocytes.17) Taken together, a reduction in the number of large adipocytes or an increase in the number of normal small adipocytes is critical for the prevention of metabolic syndrome.
Nowadays, several types of natural products are marketed as dietary supplements for obesity or metabolic syndrome. However, the efficacy and mechanistic principles underlying most of these nutritional supplements have not been fully characterized. We have screened several natural products that influence adipocyte differentiation and found that St. John’s wort (SJW), or Hypericum perforatum, promotes adipocyte differentiation. SJW, a perennial herb, has been used for the treatment of depression in several countries.18,19) SJW inhibits the serotonin and noradrenaline reuptake, and one of the active consituent of SJW is believed to be hyperforin.20,21) Though its effects on depression have been extensively studied, its influence on metabolic syndrome has not yet been elucidated. Recently, some studies have indicated that SJW affects adipocytes differentiation and inhibits insulin signaling.22,23) However, there are no studies about the effects of SJW on inflammatory signaling. Since obesity is a state of chronic low-grade inflammation leading to metabolic syndrome, it is important to study whether SJW has anti-inflammatory effects. Therefore, we investigated the role of the anti-inflammatory activity of SJW in adipocyte differentiation by using 3T3-L1 preadipocytes. Our results suggested that SJW has the potential to minimize the risk of metabolic syndrome.
SJW extract from flower tops was provided by Indena S.p.A. (Milan, Italy; serial number 28538/R1; The product sheet is shown in Supplemental Information.). Curcumin (Sigma, St. Louis, MO, U.S.A.; product number C1386, lot number 079K1756) from Turmeric, Curcuma longa, which inhibits adipocyte differentiation, was used as a reference. The effect of curcumin on adipocyte differentiation was shown in Supplemental Data.
Cell Culture and SJW Extract Treatment3T3-L1 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Nacalai Tesque; Kyoto, Japan) with 10% calf serum (CS; GIBCO; Carlsbad, CA, U.S.A.) and a penicillin and streptomycin mixed solution (Nacalai Tesque; final concentrations 100 U/mL penicillin and 100 µg/mL streptomycin), in 5% CO2 and 95% air at 37°C. The SJW extracts were dissolved in dimethyl sulfoxide (DMSO; Nacalai Tesque). Adipocyte differentiation of the cells was facilitated according to an established protocol.24) Briefly, 3T3-L1 cells were plated in 6-well plates, 24-well plates, or 35 mm dishes, and grown to confluence in DMEM with 10% CS. When the cells were confluent, they were fed DMEM supplemented with 10% fetal bovine serum (FBS; Biowest; Nuaille, France), 1 µM dexametazone (DEX; Sigma), 500 µM isobutylmethylxanthine (IBMX; Sigma), 5 µg/mL insulin (Wako Pure Chemical Industries, Ltd., Osaka, Japan), and various concentrations (10, 25, and 50 µg/mL) of SJW extract or DMSO as a vehicle control (Day 0), and cultured for 3 d. After 3 d (Day 3), the medium was replaced with DMEM containing 10% FBS, 5 µg/mL insulin, and SJW or DMSO. Two days later (Day 5), the medium was replaced with DMEM containing just SJW or DMSO, and incubated for 3 more days. These differentiated adipocytes were used in the experiments 7 d after initiation of the differentiation protocol (Day 7). 2-Chloro-5-nitrobenzanilide (GW9662) was purchased from Sigma.
Cell Viability AssayCells were cultured in 96-well plates in DMEM with 10% CS and incubated in 5% CO2 and 95% air at 37°C. One day later, the cells were fed DMEM containing 10% FBS and various concentrations (10, 25, 50, and 100 µg/mL) of SJW extract, and cultured for 72 h. The medium was then replaced with a fresh medium containing 0.5 mg/mL 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT; Nacalai Tesque). After 2 h incubation at 37°C, 150 µL of stop solution (0.04 N HCl in isopropanol) was added to each well and the formazan crystals present in the viable cells were completely dissolved by pipetting. The absorbance of each well was read at 570 nm by using the iMark microplate reader (Bio-Rad, Hercules, CA, U.S.A.). The wells that did not contain any cells served as a background for the assay.
Oil Red O StainingCells were washed with phosphate-buffered saline (PBS) and fixed with 10% formalin for 20 min, and were then washed again with PBS. The cells were stained with Oil Red O (Nacalai Tesque) (3 mg/mL Oil Red O in 99% isopropanol : water=6 : 4) for 10 min, and then the stained cells were washed three times with PBS and observed under a microscope (Nikon ECLIPSE TS100). Stained oil droplets in the cells were dissolved in isopropanol containing 4% Nonidet-P40 with gentle agitation for 5 min. The absorbance of the solution was measured at 500 nm with a spectrophotometer (UVmini1240, Shimadzu, Kyoto, Japan).
Western Blotting AnalysisAfter washing with PBS, cells were harvested and disrupted in cell lysis buffer (20 mM Tris pH 8.0, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1% Nonidet P-40, 0.1% Triton, 50 mM NaF) containing protease inhibitor cocktail (Nacalai Tesque), and were incubated for 20 min on ice. Cell debris was removed by centrifugation at 12000×g for 10 min at 4°C, and the supernatant was carefully separated. The protein concentration of the supernatant was measured by the Pierce bicinchoninic acid (BCA) protein assay (Thermo Scientific), and equal concentrations of the extracted proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The separated proteins were transferred to a polyvinylidene fluoride membrane (Immobilon-P, pore size 0.45 µm, Millipore, Billerica, MA, U.S.A.) equilibrated with methanol, using an XCell II™ Blot Module (Invitrogen, Carlsbad, CA, U.S.A.) at 25 V for 1–2 h. The membrane was blocked with 5% skim milk (Nacalai Tesque) in Tris-buffered saline with 0.1% Tween-20 (TBS-T) for 30 min, followed by overnight incubation at 4°C with primary antibody in TBS-T containing 2.5% skim milk. Anti-adiponectin/Acrp30 (R&D Systems, Minneapolis, MN, U.S.A.), anti-actin clone C4 (Millipore), anti-PPARγ (81B8), anti-IκBα, and anti-Phospho-IκBα (Ser32) (14D4) (Cell Signaling Technology, Danvers, MA, U.S.A.) were used as primary antibodies. After washing three times with TBS-T for 5 min, the membrane was incubated for 1 h at room temperature with horseradish peroxidase (HRP)-conjugated anti-goat, anti-mouse, or anti-rabbit IgG secondary antibodies (Jackson Immuno Research, West Grove, PA, U.S.A.) in TBS-T containing 2% skim milk. After washing three times with TBS-T for 5 min, the protein bands on the membrane were detected by a chemiluminescence method using an Immobilon™ Western Chemiluminescent HRP substrate (Millipore) or Amersham™ ECL™ Prime Western blotting Detection Reagent (GE Healthcare, Buckinghamshire, U.K.).
RNA Isolation and Quantification of mRNATotal RNA was isolated using RNeasy Mini Kits (QIAGEN; Valencia, CA, U.S.A.) following the manufacturer’s instructions. The cDNA was constructed from 1.7 µg of total RNA. The reverse transcriptase reaction was performed using a High-Capacity cDNA Reverse Transcription Kit (Life Technologies, Gaithersburg, MD, U.S.A.). The real-time polymerase chain reaction (PCR) with the specific primers and SYBR green dye was carried out using an Applied Biosystems™ 7000 Real-Time PCR System (Life Technologies). The forward (F) and reverse (R) primer sequences were as follows, Adiponectin: 5′-ATG GCA GAG ATG GCA CTC CT-3′ (F), 5′-CCT TCA GCT CCT GTC ATT CCA-3′ (R); IL-6: 5′-AGT TGC CTT GGG ACT GAT-3′ (F), 5′-TCC ACG ATT TCC CAG AGA AC-3′ (R); MCP-1: 5′-CCA CTC ACC TGC TGC TAC TCA T-3′ (F), 5′-TGG TGA TCC TCT TGT AGC TCT CC-3′ (R); Ribosomal protein L32 (RiboL32) 5′-GAA ACT GGC GGA AAC CCA-3′ (F), 5′-TGG TGA TCC TCT TGT AGC TCT CC-3′ (R). The PCR conditions were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 60°C for 1 min.
Statistical AnalysisThe data are expressed as mean values±S.D. Statistical analysis was performed by using Prism software (GraphPad Software, San Diego, CA, U.S.A.). Differences between two groups were analyzed by unpaired two-tailed t-tests. Data including more than two groups were analyzed by one-way ANOVA followed by the Tukey’s multiple comparison test. The significance level was set at p<0.05.
The viability of the 3T3-L1 preadipocytes in response to SJW treatment was determined by an cell viability assay in order to determine the appropriate dosage of SJW for subsequent assays. The 3T3-L1 preadipocytes were incubated with various concentrations (10, 25, 50, and 100 µg/mL) of SJW for 72 h. As shown in Fig. 1, SJW did not show significant cytotoxic effects in these cells up to a 100 µg/mL dose. Based on these results, we used SJW extracts at 10, 25, and 50 µg/mL concentrations in this study.
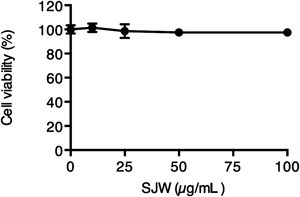
The cell viability was determined by MTT assay 72 h after incubation with the different concentrations of SJW extract, and the results are presented as the percent change in the number of viable cells relative to vehicle control (0.1% DMSO). Data are presented as mean±S.D., n=4.
We assessed the effects of SJW on the adipocyte differentiation of 3T3-L1 cells. Seven days after the induction of adipogenesis with or without SJW treatment, mature adipocytes were detected by Oil Red O staining, which indicated the intracellular lipid accumulation. Adipocyte differentiation was enhanced at 25 and 50 µg/mL of SJW (Fig. 2A). In addition, stained cells that were cultured with SJW appeared to be smaller than the controls. These cells were dissolved, and the amount of Oil Red O was quantified. Treatment with 25 and 50 µg/mL SJW extract significantly increased the lipid accumulation in the cells (Fig. 2B).
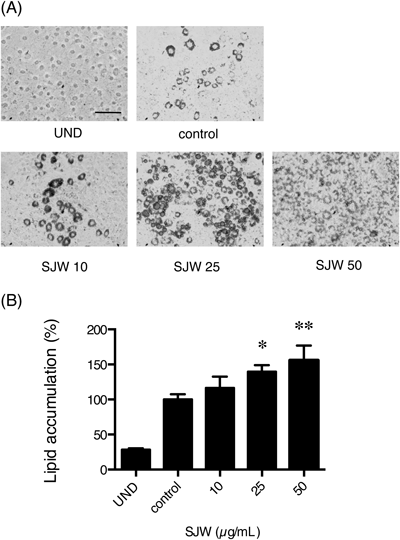
Seven days after the induction of adipocyte differentiation, in the presence of the specified concentrations of SJW extract, the differentiated cells were stained with Oil Red O. (A) Representative images of Oil Red O-stained mature 3T3-L1 adipocytes treated with various concentrations of SJW extracts. UND indicates undifferentiated cells. Scale bar is equivalent to 200 µm. (B) Quantification of lipid accumulation in differentiated 3T3-L1 adipocytes. Lipid accumulation was determined by the quantification of Oil Red O in the cells by measuring their absorbance at 500 nm. Lipid accumulation is indicated as the percent change relative to vehicle control (0.1% DMSO). Data are presented as mean±S.D., n=3. Statistical significance of differences was determined by one-way ANOVA followed by Tukey’s multiple comparison test. * indicates p<0.05 and ** indicates p<0.01 compared to control.
Then, we analyzed the effect of SJW on the expression of PPARγ in differentiated 3T3-L1 cells by Western blotting (Fig. 3A). SJW increased PPARγ expression in a dose-dependent manner, suggesting that SJW enhanced adipocyte differentiation. Our results indicated that SJW also increased the adiponectin expression (Fig. 3B). Figure 3C shows that the increase by SJW already occurred on Day 5 after the induction of differentiation. We also confirmed that SJW increased mRNA (Fig. 3D) and extracellular levels (Fig. 3E) of adiponectin. In addition, the effects of GW9662, a PPARγ antagonist, on the expression of adiponectin in the presence or absence of SJW were investigated (Fig. 3F). GW9662 decreased the adiponectin expression in the absence of SJW, but not in the presence of SJW.
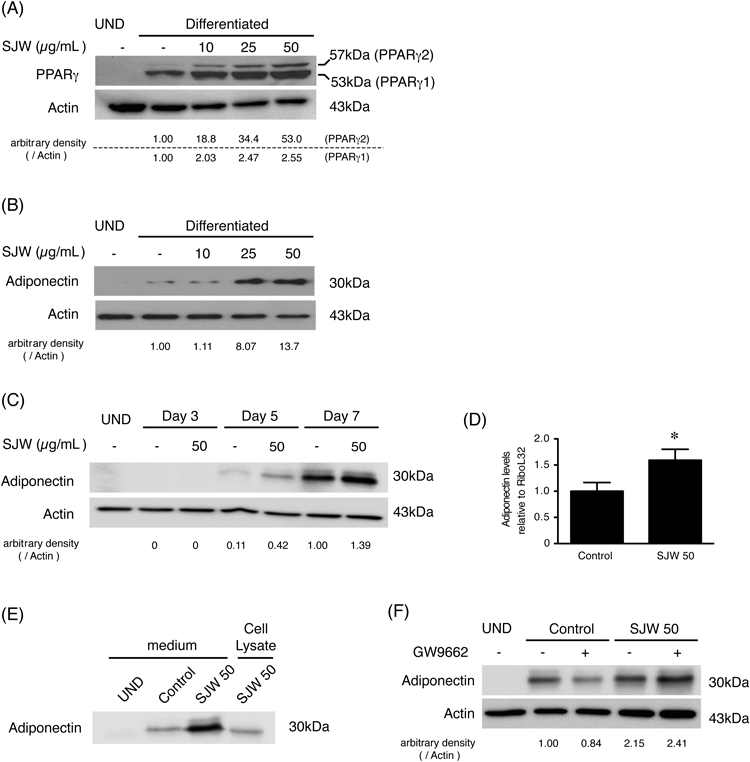
Proteins or total RNAs were extracted from 3T3-L1 cells treated with specified concentrations of SJW extract. The extractions were performed on Day 7 of adipocyte differentiation induction unless the day is indicated. UND indicates undifferentiated cells. (A and B) Adiponectin and PPARγ expression was analyzed and normalized with the expression of actin. (D) Quantitative PCR analysis of adiponectin mRNA expression of 3T3-L1 cells without (Control) and with 50 µg/mL SJW (SJW 50) treatment. The mRNA expression was quantified by real-time PCR and normalized with RiboL32. Data are presented as mean±S.D., n=3. Statistical significance of differences was determined by the Student’s t-test. * indicates p<0.05. (E) Adiponectin levels in 20 µL of 2 mL of culture medium in the absence (Control) or presence of 50 µg/mL SJW (SJW 50), and 10 µg of cell lysate was analyzed by Western blotting. (F) Effects of GW9662, PPARγ antagonist, on the expression of adiponectin in the presence of SJW. GW9662 (10 µM) was added 1h before 50 µg/mL SJW treatment.
SJW induced an increase in the expression of adiponectin, an anti-inflammatory adipokine, in the adipocytes (Fig. 3), which suggested that SJW might have anti-inflammatory effects. To further validate this hypothesis, we analyzed the activity of NF-κB. Then the effects of SJW extracts on the expression of IκBα and P-IκBα after stimulating the cells with the inflammatory cytokine TNF-α was investigated (Fig. 4A). TNF-α was added to the cells on Day 7 after the induction of adipocyte differentiation either in the absence or presence of SJW. In the absence of SJW, the addition of TNF-α to mature 3T3-L1 adipocytes dramatically decreased IκBα expression at 3 h, and this was followed by a gradual increase in the expression of IκBα, which did not reach the control levels even at 9 h after TNF-α stimulation. However, in the presence of SJW, the TNF-α-induced decrease in IκB expression was partially attenuated. Moreover, the TNF-α-induced phosphorylation of IκBα was decreased by SJW, indicating that SJW inhibits IκB phosphorylation and in turn prevents the degradation of IκB. These results suggest that SJW is capable of inhibiting the NF-κB activation induced by TNF-α, and consequently prevents the transcriptional induction of inflammatory cytokines.
Finally, we demonstrated that SJW is able to inhibit the TNF-α-induced expression of inflammatory cytokines in the adipocytes. The expression level of TNF-α-induced inflammatory cytokines that are regulated by NF-κB, such as IL-6 or MCP-1, in the presence and absence of SJW (Fig. 4B) were compared. The addition of TNF-α increased both IL-6 and MCP-1 mRNA levels in mature 3T3-L1 cells. As expected, SJW extracts significantly inhibited the TNF-α-mediated induction of these cytokines.
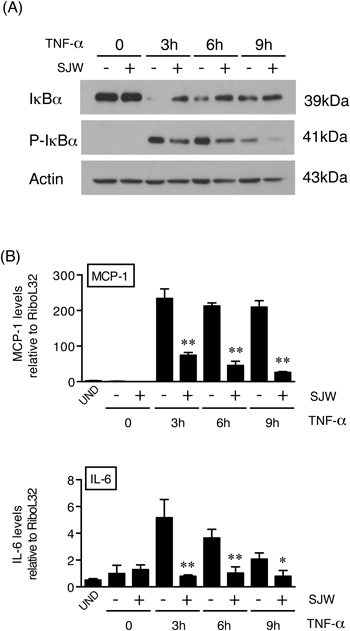
TNF-α was (10 ng/mL) added to the cells on day 7 of adipocyte differentiation induction in the absence or presence of SJW (− indicates treatment with 0.1% DMSO as a vehicle control; + indicates treatment with 50 µg/mL of SJW extract), and protein or total RNA were extracted at specified time-points. (A) Western blot analysis of IκBα and P-IκBα expression. The immunoblots were normalized with the expression of actin. (B) Quantitative PCR analysis of IL-6 and MCP-1 mRNA expression. The mRNA expression was quantified by real-time PCR and normalized with RiboL32. Data are presented as mean±S.D., n=3. Statistical significance of differences was determined by the Student’s t-test. * indicates p<0.05 and ** indicates p<0.01 compared to vehicle control.
SJW extracts have been used for the treatment of depression in many countries.18,19) Hence, its effects on depression have been evaluated in several studies. Recently, some studies have indicated that SJW affects adipocytes differentiation and inhibits insulin signaling.22,23) However, there are no studies about the effects of SJW on inflammatory signaling. Since obesity is a state of chronic low-grade inflammation leading to metabolic syndrome, it is important to study whether SJW has anti-inflammatory effects. Our results suggest that SJW extracts might be useful in the prevention of metabolic syndrome by inhibiting inflammatory. Epidemiological studies indicate that there is a close association between the incidence of depression and diabetes.25) A meta-analysis study indicated that the presence of diabetes doubles the odds of comorbid depression.26) In this study, the authors suggested that better recognition and better treatment of depression could also improve the clinical outcome in a substantial portion of patients with diabetes. Hence, it is intriguing to note that the SJW extracts that have anti-depression effects could potentially influence metabolic syndrome and diabetes.
Here, we showed that SJW, a perennial herb, enhanced adipocyte differentiation and increased adiponectin expression via the PPARγ pathway. We also showed that SJW prevented TNF-α-mediated pro-inflammatory cytokine, such as MCP-1 and IL-6, induction by the inhibition of NF-κB activation (Fig. 5). Adiponectin protects against inflammation by inhibiting NF-κB activity and obesity-linked insulin resistance.7,8,27,28) Since obesity is a state of chronic low-grade inflammation leading to metabolic syndrome, it is important to prevent inflammation in order to prevent the consequent development of metabolic syndrome. Although the associated signaling pathways need to be clarified, these results suggest that SJW extracts have an anti-inflammatory effect by promoting adiponectin expression.
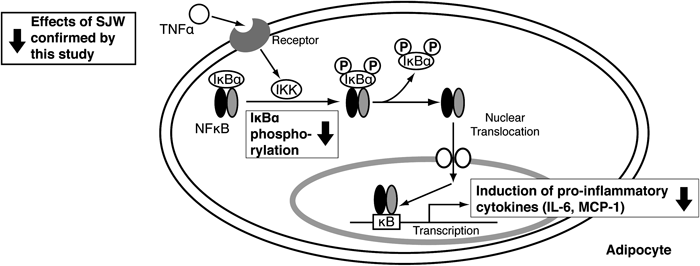
SJW extracts contain hyperforin, hypericin, tannin, various flavonoids, and other active principles.18) Hyperforin is thought to be the major constituent that is responsible for the anti-depressive effects.29) Although our study demonstrated the anti-inflammatory effect of SJW, the particular SJW constituents that are responsible for PPARγ pathway activation are unknown. Thiazolidinediones, which are synthetic insulin-sensitizing drugs used for treating insulin resistance and type 2 diabetes, are well known agonists of PPARγ.30,31) Both in vivo and in vitro studies indicated that thiazolidinediones increase the number of small adipocytes by promoting differentiation, and decrease the number of large adipocytes by inducing apoptosis.32,33) The increased small adipocytes facilitate anti-inflammatory effects by secreting adiponectin, and by regulating glucose and lipid metabolism.12–14) Our study indicated that SJW treatment increased adiponectin expression in differentiated 3T3-L1 adipocytes. The effects of SJW extracts on adipocyte differentiation seem to be similar to those of thiazolidinediones. However, PPARγ antagonist did not diminish the increase of adiponectin expression enhanced by SJW treatment (Fig. 3F). This result suggests that SJW enhanced adipocyte differentiation via both PPARγ-dependent and -independent mechanism such as AMPK-Sirt1 pathway.34) We need further investigations to be clear this mechanism.
Amini et al.22) reported that the extracts from the leaves and flowers of SJW inhibited adipocyte differentiation. They showed that the PPARγ and adiponectin expression levels were decreased in the presence of SJW extracts in mature 3T3-L1 adipocytes. Interestingly, the extracts of roots did not affect adipocyte differentiation in their study, suggesting that the effects of SJW depend on the tissue used. Here, we also employed commercially available SJW extracts from the flower tops. Hence, the composition as well as the concentration of the effective constituents in our SJW extracts likely differed from those used in other studies. The differences in the constituents might be responsible for the induction of apoptosis at higher concentration rather than the inhibition of adipocyte differentiation. We need to identify the constituent responsible for these effects in further study.
In conclusion, our study demonstrated that SJW extracts promote adipocyte differentiation and increase adiponectin expression via the PPARγ pathway. In addition, SJW might have an anti-inflammatory effect by preventing the induction of pro-inflammatory cytokines. These results suggest that SJW extracts have the potential to prevent metabolic syndrome.
This work was supported by an encouragement grant from Mukogawa Women’s University. We are grateful to Dr. Naomi Yasui and Dr. Sachiko Juman for helpful discussions and technical advice, and to Chieko Yokoyama, Yuka Tatara and all of the research students in our laboratory for helping us complete the experiments.