2014 Volume 37 Issue 8 Pages 1308-1314
2014 Volume 37 Issue 8 Pages 1308-1314
O-Linked β-N-acetylglucosamine-modification (O-GlcNAcylation) is a reversible, post-translational, and regulatory modification of nuclear, mitochondrial, and cytoplasmic proteins that is responsive to cellular stress. However, the role of O-GlcNAcylation in the induction of heat shock proteins (Hsps) by arsenite remains unclear. We used O-(2-acetamido-2-deoxy-D-glucopyranosylidene) amino N-phenyl carbamate (PUGNAc), an inhibitor of O-GlcNAcase, and glucosamine (GlcN), an enhancer of the hexosamine biosynthesis pathway, or O-GlcNAc transferase (OGT) short interfering RNA (siRNA) to enhance or suppress cellular O-GlcNAcylation levels, respectively, in HeLa cells. The exposure to arsenite increased O-GlcNAcylation and Hsp 70 levels in HeLa cells. However, the pre-treatment with PUGNAc or GlcN, which enhanced O-GlcNAcylation levels, decreased the arsenite-induced expression of Hsp 70. The pre-treatment with OGT siRNA, which suppressed O-GlcNAcylation levels, did not affect the induction of Hsp 70. We then examined the effects of O-GlcNAcylation on the nuclear translocation and phosphorylation of heat shock factor 1 (HSF1), and found that neither the nuclear translocation nor phosphorylation of HSF1 was regulated by O-GlcNAcylation. Finally, Hsp 70 mRNA expression was induced by arsenite, whereas the addition of PUGNAc slightly suppressed its induction. These results indicate that O-GlcNAcylation is related to arsenite-induced Hsp 70 expression, and demonstrated that hyper-O-GlcNAcylation inhibited the induction of Hsp 70 via transcriptional factors instead of HSF1.
O-Linked β-N-acetylglucosamine (O-GlcNAc) modifications of serine and threonine residues have been shown to regulate dynamic cellular functions such as cell morphogenesis, cell signaling, apoptosis, and transcription.1–4) It is a reversible modification that is similar to phosphorylation, and previous studies have demonstrated that O-GlcNAc cycling is involved in various pathological conditions and diseases.4,5) O-GlcNAcylated proteins also function as regulators of cellular stress responses. O-GlcNAc levels of various subcellular proteins were reported to vary under the conditions such as glucose deprivation,6) UV-irradiation, heat shock, and oxidative stress.7) Taken together, O-GlcNAcylation may be involved in stress responses; however, this involvement has not yet been elucidated in detail.
Heat shock proteins (Hsps) are a set of highly conserved proteins produced in response to physiological and environmental stresses, and have been shown to play a role in protecting cells from stress-induced damage by preventing protein degeneration and/or repairing such damage.8) Mammalian Hsps have been classified into several families on the basis of their apparent molecular weights and functions, such as Hsp 105/101, Hsp 90, Hsp 70, Hsp 60, Hsp 40, and Hsp 27. The Hsp 70 family is a major Hsp group that has been characterized in the most detail, and several transcription factors of Hsp 70 expression have been identified, such as heat shock factor 1 (HSF1)9–11) and Sp1.12)
Arsenic (As) is a naturally occurring element, and environmental or occupational exposure to arsenic may result in both acute and chronic toxic effects in humans. The toxicity of arsenite (As3+) has been attributed to its affinity for sulfhydryls.13) A number of sulfhydryl-containing proteins and enzymes may be altered following their exposure to arsenite. Glutathione, which is the major cellular non-protein thiol-reductant and one of the main protectors against reactive oxygen species (ROS)-induced damage, has also been shown to be affected following its exposure to arsenite.14) A previous study demonstrated that arsenite caused DNA damage in keratinocytes through the generation of hydroxyl radicals.15) Taken together, these findings show that arsenite exposure can be regarded as an oxidative stress. O-GlcNAcylation may be involved in the cellular response to arsenite because exposure to it has been shown to increase cellular O-GlcNAcylation levels in COS-7 cells.7) However, the mechanisms responsible remain unclear. We here examined the effects of O-GlcNAcylation on the induction of Hsp 70 caused by exposure to arsenite, and discussed the role of O-GlcNAcylation in cell responses to oxidative stress.
An inhibitor of O-GlcNAcase (OGA), O-(2-acetamido-2-deoxy-D-glucopyranosylidene) amino N-phenyl carbamate (PUGNAc), and glucosamine (GlcN) were purchased from Toronto Research Chemicals Inc. (North York, ON, Canada) and Sigma-Aldrich (St. Louis, MO, U.S.A.), respectively. Sodium arsenite was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). The antibodies used in this study were anti-Hsp 70 (610607, BD Biosciences, Sparks, MD, U.S.A.), anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (6C5, Millipore Corporation, Billerica, MA, U.S.A.), anti-O-GlcNAc (CTD110.6, Covance Laboratories, Madison, WI, U.S.A. and HGAC85, Pierce Biotechnology, Rockford, IL, U.S.A.), anti-α-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), anti-structural maintenance of chromosomes 1 (SMC1) (Abcam, Cambridge, U.K.), anti-HSF1 (Cell Signaling Technology, Beverly, MA, U.S.A.), and anti-O-GlcNAc transferase (OGT) (Abcam). The peroxidase-conjugated secondary antibodies used were anti-mouse immunoglobulin G (IgG), anti-mouse IgM, and anti-goat IgG (Santa Cruz Biotechnology), and anti-rabbit IgG (Cell Signaling Technology). Phos-tag™ acrylamide was purchased from Wako Pure Chemical Industries, Ltd.
Cell Cultures and TreatmentHeLa cells were purchased from the Riken Bioresource Center Cell Bank (RCB 0007, Tsukuba, Japan). The cell line was cultured in minimum essential medium (MEM) (Invitrogen, Carlsbad, CA, U.S.A.) supplemented with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, Rockford, IL, U.S.A.) and 50 U/mL penicillin-50 µg/mL streptomycin, and was subcultured twice per week.
Cells were seeded onto 10-cm, 6-cm, or 3.5-cm dishes at 1.7 to 1.8×104 cells/cm2 and sodium arsenite was added to the cell culture 24 h after seeding. Cells were exposed to arsenite for 5 h at 37°C and subjected to assays. In experiments involving the treatment with PUGNAc or GlcN, cells were incubated with reagents from 1 h before the addition of sodium arsenite.
RNA Interference (RNAi)The RNAi of OGT was performed using Lipofectamine™ RNAiMAX (Invitrogen) according to the manufacturer’s instructions (Reverse transfection). A total of 30 pmol RNAi duplex diluted with 500 µL Opti-MEM I medium (Invitrogen) was mixed with 4 µL Lipofectamine™ RNAiMAX in each well in the 6-well plates. After being incubated at room temperature, HeLa cells were seeded in the wells at 1.5×105 cells in MEM (Invitrogen) without antibiotics. Cells were incubated for 24 h at 37°C under a 5% CO2 atmosphere and subjected to assays. RNAi duplexes designed against the human OGT sequence were purchased from Stealth™ RNAi (Invitrogen): OGT1 (5′-GCC CUC UGU UCA ACA CCA AAC AAU A-3′), OGT2 (5′-UAU UGU UUG GUG UUG AAC AGA GGG C-3′), OGT3 (5′-GCA GAA GCA GUU AUU GAA AUG AUU A-3′). An equal mixture of OGT1, OGT2, and OGT3 was used. BLOCK-iT™ Alexa Fluor® red fluorescent oligo was used as a control oligo in mock experiments.
Subcellular FractionationCytoplasmic and nuclear fractions were separated using a ProteoExtract™ Subcellular Proteome Extraction Kit (Merck Millipore, Billerica, MA, U.S.A.) according to the manufacturer’s instructions. The purity of cytoplasmic and nuclear fractions was confirmed by immunoblot analysis using antibodies against cytoplasmic and nuclear markers (cytoplasm, GAPDH; nucleus, SMC1). In immunoprecipitation experiments, samples from the cytoplasmic and nuclear fractions were subjected to immunoprecipitation using a specific antibody (anti-HSF1) or control IgG (rabbit IgG) (Vector Laboratories, Burlingame, CA, U.S.A.).
Immunoblot AnalysisCell lysates were treated with sample buffer consisting of 50 mM Tris–HCl (pH 6.8), 2% sodium dodecyl sulfate (SDS), 10% glycerol, 1% 2-mercaptoethanol, and 0.01% bromophenol blue, followed by boiling for 10 min. After protein concentrations were determined in each sample using a protein assay kit (Bio-Rad Laboratories, Hercules, CA, U.S.A.), equal amounts of protein were loaded (10–40 µg of protein/lane) into individual lanes, separated on 6% or 10% gels via SDS-polyacrylamide gel electrophoresis (PAGE), and transferred onto polyvinylidene difluoride (PVDF) membranes (Immobilon-P, Merck Millipore). In the case of phos-tag SDS-PAGE,16) we used 20 µM Mn2+-phos-tag in 12% acrylamide gels, and performed electrophoresis in a manner identical to traditional SDS-PAGE. After electrophoresis, Mn2+ ions were removed from phos-tag gels by incubating twice with transfer buffer containing 10 mM ethylenediaminetetraacetic acid (EDTA) for 10 min. The proteins were transferred to PVDF membranes for 1.5 h at 100 V. Protein transfer and the uniformity of protein loading were monitored using reversible Ponceau S staining. The membranes were blocked with 1% or 5% bovine serum albumin (BSA) in TBS-T (Tris-buffered saline and 0.05% Tween-20) (for anti-O-GlcNAc antibodies), or 5% skim milk in TBS-T (for other antibodies) for 1 h at room temperature; membranes were then incubated with primary antibodies diluted with blocking solution overnight at 4°C. After three washes with TBS-T for 5 min, the membranes were then incubated with peroxidase-conjugated secondary antibodies diluted with blocking solution for 2 h at room temperature. After another three washes with TBS-T and four rinses with milli-Q water, the immunoreactive bands were visualized using ECL plus (Thermo Fisher Scientific, Rockford, IL, U.S.A.) and Hyperfilm™ ECL (GE Healthcare, Bucks, U.K.) according to the manufacturer’s instructions. Densitometric analyses were performed using ImageJ 1.38× software (NIH, MD, U.S.A.).
ImmunoprecipitationThe immunoprecipitation of fractionated samples was performed using Dynabeads® Protein G (Invitrogen) according to the manufacturer’s instructions. Immunoprecipitates were collected using a magnet, suspended in SDS-sample buffer, and heated at 95°C for 2 min. The immunoprecipitates were then subjected to SDS-PAGE and immunoblot analysis as described above.
Quantitative Real-Time Polymerase Chain Reaction (PCR)Total RNA from HeLa cells was isolated using the RNeasy mini kit (Qiagen Co., Victoria, Australia) according to the manufacturer’s instructions. The quality and quantity of total RNA isolated from all samples were determined using Bioanalyzer series II (Agilent Technologies, Santa Clara, CA, U.S.A.) with the RNA6000 nano kit (Agilent Technologies). After reverse transcription with the SuperScript® VILO™ cDNA synthesis kit (Invitrogen), the resulting cDNA was used for quantitative real-time PCR. Real-time PCR was performed using the StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA, U.S.A.). Amplification was performed in a final volume of 20 µL using the KAPA Probe fast ABI Prism qPCR kit (KAPA Biosystems, Cape Town, South Africa). TaqMan®_Gene Expression Assays (a primer of Hsp70; HSPA1A, Hs00359163-s1) were purchased from Applied Biosystems. As an internal standard, 18S ribosomal RNA (rRNA) (TaqMan® Ribosomal RNA Control Reagents, No. 4308329; Applied Biosystems) was determined in the real-time PCR assay for each cDNA sample. All other reagents were of the highest quality available.
Statistical AnalysisExperimental data are expressed as means±S.D. A paired t-test was performed to compare two groups. A p-value of less than 0.05 was considered significant.
We examined alterations in cellular O-GlcNAc levels by the addition of arsenite (Fig. 1, Supplementary Fig. S1). Cultured cells were exposed to varying concentrations of sodium arsenite for 5 h, and collected and analyzed by immunoblotting using an anti-O-GlcNAc antibody. PUGNAc (50 µM) or GlcN (10 mM) were added to cells 1 h before the addition of arsenite. Since PUGNAc is an inhibitor of OGA,17) which removes the O-GlcNAc residue from proteins,18) and GlcN supplies glucosamine-6-phosphate, which is a product of glutamine:fructose-6-phosphate aminotransferase, the rate-limiting enzyme of the hexosamine biosynthesis pathway,19) the addition of either reagent resulted in an enhancement in cellular O-GlcNAcylation levels. Although PUGNAc is a powerful inhibitor of OGA (Ki=50 nM), it is also reported to inhibit lysosomal β-hexosaminidase.20) Therefore, besides PUGNAc, we used GlcN, which enhanced cellular O-GlcNAcylation in a different manner from PUGNAc. The effects of these reagents on the cell viability were shown in Supplementary Fig. S2. The addition of GlcN decreased viability of HeLa cells, while PUGNAc did not affect significantly under our experimental conditions.

PUGNAc (50 µM) or GlcN (10 mM) was added to HeLa cell cultures 1 h before the arsenite exposure at each concentration. After 5 h of arsenite exposure, cells were collected and assayed by Western blotting using anti-O-GlcNAc and α-tubulin antibodies, as described in Materials and Methods. Typical immunoblot images following staining with anti-O-GlcNAc or α-tubulin antibodies are shown.
The addition of arsenite to cell cultures increased cellular O-GlcNAcylation in a dose-dependent manner in control (Fig. 1). Although cellular O-GlcNAcylation increased in the presence of PUGNAc or GlcN, it did not increase further following exposure to arsenite, which suggested that cellular O-GlcNAcylation had already reached a plateau in the presence of PUGNAc or GlcN.
Induction of Hsp 70 by the Addition of Arsenite and the Effects of PUGNAc or GlcN on the Induction of Hsp 70Arsenite is toxic and induces various stress responses in cells, such as the induction of Hsp 70, a stress responsive protein.21) Thus, we examined the relationship between O-GlcNAcylation levels and the induction of Hsp 70 caused by arsenite. Arsenite increased the expression of Hsp 70 in a dose-dependent manner, and reached a maximum at 50 µM (Fig. 2). However, the pre-increase in O-GlcNAcylation by the addition of PUGNAc or GlcN reduced the induction of Hsp 70 by arsenite (Fig. 2).

PUGNAc (50 µM) or GlcN (10 mM) were added to HeLa cell cultures 1 h before arsenite exposure at each concentration. After 5 h of exposure, cells were collected and assayed by Western blotting using anti-Hsp 70 and α-tubulin antibodies, as described in Materials and Methods. (a) Typical immunoblot images following staining with anti-Hsp70 or α-tubulin antibodies are shown. (b) Relative ratios of the band intensities of Hsp 70 to corresponding α-tubulin. Data represent the means±S.D. * p<0.05 vs. control (80 µM arsenite) n=3, ** p<0.05 vs. control (50 µM arsenite), n=5.
The O-GlcNAcylation of proteins is regulated by two enzymes, O-GlcNAc transferase (OGT), which transfers the GlcNAc residue onto the substrate proteins,22) and O-GlcNAcase (OGA). We examined whether a reduction in cellular O-GlcNAcylation levels with OGT siRNA affected the induction of Hsp 70 by arsenite. The OGT siRNA treatment decreased the expression of OGT, resulting in a significant reduction in O-GlcNAcylation levels (Fig. 3a). Under these conditions, the addition of arsenite increased the expression of Hsp 70, similar to the mock samples (Figs. 3a, b).

OGT-specific siRNA or control oligos were transfected into HeLa cells as described in Materials and Methods. The cells were incubated for 24 h at 37°C under a 5% CO2 atmosphere, and exposed to 50 µM arsenite followed by Western blotting using anti-O-GlcNAc, anti-OGT, anti-Hsp70, and anti-α-tubulin antibodies. (a) Typical immunoblot images following staining with anti-O-GlcNAc, anti-OGT, anti-Hsp 70, and anti-α-tubulin antibodies are shown. (b) Relative ratios of the band intensities of Hsp 70 to corresponding α-tubulin. Data represent the means±S.D., n=4.
HSF 1 is a transcription factor of Hsp 70 and is associated with the expression of the Hsp 70 protein. It has been shown to form a trimer when stimulated by various stresses and translocates from the cytoplasm into the nucleus.9) Thus, changes in cellular O-GlcNAcylation levels may alter the nuclear translocation of HSF1 and eventually Hsp 70 expression.
First, we examined the effects of PUGNAc on the translocation of HSF1. HSF1 was present in the cytoplasm without arsenite, and then translocated into the nucleus with the addition of 50 µM arsenite (Fig. 4). The molecular weight of HSF1 in the nuclear fraction with arsenite was slightly increased. We examined whether the PUGNAc treatment affected molecular weight changes in and the translocation of HSF1. The results obtained showed that PUGNAc did not affect either molecular weight changes in or the localization of HSF1 with or without arsenite, which indicated that cellular O-GlcNAcylation levels did not affect the nuclear translocation of HSF1 (Fig. 4).

After arsenite exposure at 50 µM for 5 h, cells were collected and fractionated using a ProteoExtract™ Subcellular Proteome Extraction Kit according to the manufacturer’s instructions. Cytoplasmic and nuclear fractions were assayed by Western blotting using anti-HSF1, GAPDH, and SMC1 antibodies.
As described above, the increase induced in O-GlcNAc levels by the PUGNAc treatment did not affect molecular weight changes in and the translocation of HSF1. HSF1 was previously shown to be phosphorylated at several serine and threonine residues, from 19 potential phosphorylation sites, by stresses such as heat shock.9) Therefore, the molecular shift in HSF1 by the arsenite treatment may be due to hyper-phosphorylation. Because many phosphorylated proteins are subjected to O-GlcNAcylation,23) it is possible that HSF1 is also subject to O-GlcNAcylation. However, whether HSF1 is an O-GlcNAc-modified protein remains controversial. HSF1 was previously shown to be modified by O-GlcNAcylation,24–26) whereas at least one study reported that it was not.27) Thus, we examined whether HSF1 was modified by O-GlcNAcylation in HeLa cells using immunoprecipitation experiments with an anti-HSF1 antibody and immunoblots with anti O-GlcNAc antibodies. The results obtained demonstrated that both high and low molecular weight HSF1 were not modified by O-GlcNAc residues (Fig. 5). These results are consistent with the findings of Kazemi et al. in murine embryonic fibroblasts (MEFs) and COS-7 cells,27) but not with those of Wischmeyer,24,25) in which HSF1 was shown to be O-GlcNAcylated in lung homogenates from septic mice and cultured MEFs.
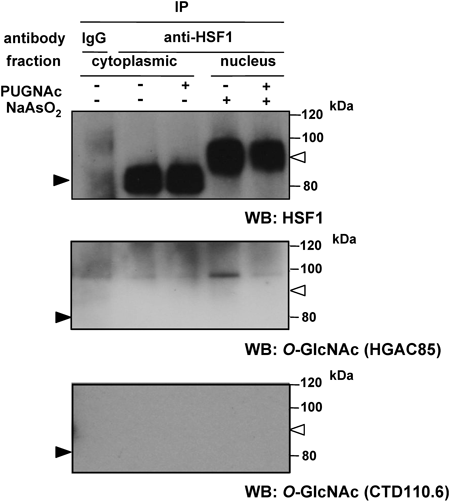
Cytoplasm and nuclei were fractionated from the cells after exposure to arsenite at 50 µM, respectively. The cytoplasmic and nuclear fractions were immunoprecipitated using an anti-HSF1 antibody or control IgG, and were then assayed by Western blotting using anti-HSF1 and anti-O-GlcNAc antibodies. Closed and open triangles indicate bands of low and high molecular weight HSF1, respectively.
HSF 1 is phosphorylated at several serine and threonine residues, and the kinases involved in HSF1 phosphorylation are known to be O-GlcNAcylated proteins.28) Therefore, the O-GlcNAcylation levels of these kinases may affect their kinase activities and also the phosphorylation of HSF1. In order to investigate whether the phosphorylation of HSF1 was affected by cellular O-GlcNAcylation levels, we examined the effects of PUGNAc or OGT siRNA on the phosphorylation of HSF1 using phos-tag Western blotting. A dinuclear metal complex, the phos-tag, which is incorporated into a polyacrylamide gel matrix prior to SDS-PAGE, promotes the physical separation of phospho-proteins proportional to their phosphorylation stoichiometry. A single protein can be separated into multiple bands, each corresponding to a different phospho-state.16) Since there are potential 19 phosphorylation sites in HSF1,9) the bands of phosphorylated HSF1 appeared as a ladder depending on the phosphorylation states of each component (Fig. 6a). These results showed that either the addition of PUGNAc or OGT siRNA treatment did not affect the phos-tag pattern of phosphorylated HSF1 following the arsenite exposure (Fig. 6a). We estimated the ratios of the intensities of phosphorylated or non-phosphorylated HSF1 to total HSF1. The results obtained showed that the ratio of phosphorylated HSF1 was not influenced by changes in cellular O-GlcNAcylation levels (Figs. 6a, b), which indicated that the phosphorylation of HSF1 did not depend on cellular O-GlcNAcylation levels.

PUGNAc (50 µM) was added to HeLa cells 1 h before the arsenite exposure at 50 µM. OGT- or control-siRNA were transfected into HeLa cells 24 h before the arsenite exposure at 50 µM. After the arsenite exposure for 5 h, cells were collected and assayed by phos-tag Western blotting using an anti-HSF1 antibody. (a) Typical phos-tag Western blotting using an anti-HSF1 antibody. Closed and open triangles indicate bands of non-phosphorylated and phosphorylated HSF1, respectively. (b) Relative ratios of non-phosphorylated HSF1 (closed bar) or phosphorylated HSF1 (open bar) bands to the total band intensities of HSF1 were calculated.
Total RNA was isolated from HeLa cells treated or non-treated with PUGNAc or arsenite. Hsp 70 mRNA expression was induced by arsenite, whereas the addition of PUGNAc suppressed slightly its induction (Fig. 7). These results suggest that the enhancement in cellular O-GlcNAcylation slightly reduced the induction of Hsp 70 mRNA.

PUGNAc (50 µM) was added to HeLa cells 1 h before the arsenite exposure at 50 µM. Cells were collected 5 h after the arsenite exposure. Hsp 70 mRNA expression was assayed as described in Materials and Methods. Data represent the mean±S.E., n=5.
Various stresses such as environmental stress, oxidative stress, hyperthermia, nutrient stress, and X-irradiation have been reported to increase cellular O-GlcNAcylation levels in various cellular systems.6,7,24,26,29,30) We found an enhancement in cellular O-GlcNAcylation levels when HeLa cells were exposed to arsenite, which indicated that O-GlcNAcylation is involved in the signaling pathways that respond to arsenite exposure.
The mechanisms underlying these acute increases in cellular O-GlcNAcylation levels by various stresses have been studied previously. The induction of Hsps, which play an important role in cell survival and as molecular chaperones, in heat stress, in particular, has been widely investigated. Zachara et al. reported that an enhancement in O-GlcNAcylation promoted the induction of Hsp 70 in COS-7 and MEFs.7) Furthermore, Wischmeyer and colleagues also showed that glutamine, which is a key substrate for the hexosamine biosynthesis pathway, promoted the induction of Hsp 70 by enhancing the O-GlcNAcylation of HSF1 in MEF cells.24,25) Gong and Jing reported similar results in LPS-treated rat cardiomyocytes.26) Taken together, the findings of several studies suggest that the induction of Hsp 70 may be enhanced by O-GlcNAcylation and that acute increases in cellular O-GlcNAcylation levels by stress may contribute to the efficient induction of Hsp 70. In contrast, Sohn et al. reported that O-GlcNAcylation did not affect the induction of Hsps in OGT-overexpressed cells (CHO-ogt).29) Majumdar et al. demonstrated that the enhanced O-GlcNAcylation of Sp1, a transcription factor of Hsp 70, prevented transcriptional activation through the reduction of phosphorylated Sp1 in liver cells (H-411E cells).31) Thus, the involvement of O-GlcNAcylation in the induction of Hsps appears to be complicated and is dependent on both the induced stresses and cellular system.
Our results indicated that acute increases in cellular O-GlcNAcylation levels occurred in arsenite-exposed HeLa cells. However, the enhancement in O-GlcNAcylation by PUGNAc or GlcN suppressed the induction of Hsp 70, whereas the reduction in O-GlcNAcylation by OGT siRNA had no influence. To clarify the involvement of O-GlcNAcylation in the induction of Hsp 70 in arsenite-exposed HeLa cells, we examined the effects of O-GlcNAcylation on the nuclear translocation of HSF1 in HeLa cells. Several previous studies demonstrated that an enhancement in O-GlcNAcylation promoted the nuclear translocation of HSF1.24–26) However, our results indicated that enhanced cellular O-GlcNAcylation by PUGNAc did not affect the translocation of HSF1. The reason for this discrepancy is currently unclear; however, one possibility is that the O-GlcNAcylation state of HSF1 in some cells may be lower than that in other cells. Whether HSF1 is an O-GlcNAc-modified protein remains controversial. HSF1 was previously shown to be modified by O-GlcNAcylation,24–26) while another study showed that it was not.27) Although we examined whether HSF1 was modified by O-GlcNAcylation in HeLa cells using immunoprecipitation with an anti-HSF1 antibody, it was not modified under our experimental conditions. Our results are consistent with those of Kazemi et al. in MEF or COS-7 cells.27) We did not find any convincing evidence for HSF1 being an O-GlcNAcylated protein in HeLa cells.
The transcriptional activity of HSF1 is known to be regulated by the phosphorylation of various kinases,9) which are O-GlcNAcylated proteins,28) We examined the effects of O-GlcNAcylation levels on the phosphorylation of HSF1. The results obtained showed that neither hyper- nor hypocellular O-GlcNAcylation levels affected the phosphorylation levels of HSF1 caused by arsenite exposure, which indicated that changes in O-GlcNAcylation levels did not affect the phosphorylation levels of HSF1. However, we also demonstrated that an enhancement in cellular O-GlcNAcylation slightly suppressed the induction of Hsp 70 mRNA by arsenite (Fig. 7). In contrast, arsenite-induced Hsp 70 protein level was inhibited by treatment of PUGNAc by more than 50% (Fig. 2b). At the present, the difference between Hsp 70 mRNA and protein levels by hyper-O-GlcNAcylation is unclear. It is known that O-GlcNAcylation regulates various steps involved in protein synthesis, such as translation and ribosome biogenesis,32) and protein degradation, such as proteasomal function33) and ubiquitination.34) Therefore, these processes in the induction of Hsp 70 may be affected by hyper-O-GlcNAcylation, e.g., inhibition of protein synthesis and/or enhancement of protein degradation, but transcriptional step may not be affected much.
Several studies have demonstrated that an acute increase in O-GlcNAcylation improves cellular survival under various stresses.7) On the other hand, O-GlcNAcylation has been implicated as a pathogenic contributor to glucose toxicity and insulin resistance,35) and causes ER stress in several types of cell cultures.36) These findings indicate that the role of O-GlcNAcylation in cellular functions may be friend or foe.37,38) Our results demonstrated that hyper-O-GlcNAcylation prevented a particular cellular response, namely the induction of Hsp 70 caused by arsenite exposure. Since the induction of Hsps is known to participate in adaptive response,39) hyper-O-GlcNAcylation might make the cells more susceptible to the repetitive arsenite exposure. Although further studies are needed to clarify the mechanisms responsible in more detail, our results suggest that O-GlcNAcylation may have adverse effects on cellular stress responses.
This study was supported in part by a Grant-in-Aid for Scientific Research (C) (No. 20590082 to Y.M.) from the Japan Society for the Promotion of Science, and by a Grant-in-Aid for Challenging Exploratory Research (No. 21659022 to T.E.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.