Abstract
The circadian clock is a cell-autonomous endogenous system that generates circadian rhythms in the behavior and physiology of most organisms. We previously reported that the harmala alkaloid, harmine, lengthens the circadian period of Bmal1 transcription in NIH 3T3 fibroblasts. Clock protein dynamics were examined using real-time reporter assays of PER2::LUC to determine the effects of harmine on the central clock in the suprachiasmatic nucleus (SCN). Harmine significantly lengthened the period of PER2::LUC expression in embryonic fibroblasts, in neuronal cells differentiated from neuronal progenitor cells and in SCN slices obtained from PER2::LUC mice. Although harmine did not induce the transient mRNA expression of clock genes such as Per1, Per2 and Bmal1 in embryonic fibroblasts, it significantly extended the half-life of PER2::LUC protein in neuronal cells and SCN slices. Harmine might lengthen the circadian period of the molecular clock by increasing PER2 protein stability in the SCN.
The circadian clock is an endogenous cell-autonomous system that generates circadian rhythms in the behavior and physiology of most organisms. The master clock in mammals is located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus,1,2) which controls most aspects of physiology such as sleep-wake cycles, body temperature, hormone secretion, blood pressure and metabolism.3,4) The circadian clock mechanism consists of a network of autoregulatory transcription-based feedback loops that drive the rhythmic expression of clock genes such as Per1, Per2 and Bmal1,5) and these cell-autonomous and self-sustained oscillators are found not only in the SCN but also in peripheral tissues as well as dissociated cells. Acute induction of the mRNA expression of clock genes plays a critical role in phase shifting the circadian clock both in cells cultured in vitro and in vivo. In addition to transcriptional feedback regulations, some essential roles of post-translational modifications such as phosphorylation and ubiquitination have been reported.6–8)
The harmala alkaloid, harmine, is a β-carboline alkaloid that was originally isolated from seeds of Peganum harmala and Banisteropsis caapi, both of which have traditionally been used in ritual and medicinal preparations of the Middle East, Central Asia and South America.9) Harmine is also found in common plant-derived foods and animal tissues,10) and it has antiplasmodial, antioxidative, antimutagenic and antigenotoxic activities, as well as antidepressant properties.11–13) Several psychiatric conditions, including depression, are closely associated with the circadian clock.14) Although we previously reported that harmine dose-dependently lengthens the circadian period of Bmal1 expression in NIH 3T3 cells,15) the effects of harmine on the master clock in the SCN remain unknown. We therefore evaluated clock protein dynamics in neuronal cells and in SCN slices by monitoring PER2::LUC bioluminescence in real-time to determine the effects of harmine on the master clock in the SCN.
MATERIALS AND METHODS
AnimalsPER2::LUC C57BL/6j mice (Jackson Laboratories, Bar Harbor, ME, U.S.A.) were housed under a 12 h light: 12 h dark cycle (lights on at 08:00) at a controlled ambient temperature of 24±1°C and provided with food and water ad libitum. Endogenous PER2 protein is fused in-frame with a luciferase reporter in this knock-in mouse, allowing real-time monitoring of PER2::LUC protein dynamics by recording bioluminescence.16) This study proceeded in accordance with the guidelines for the Care and Use of Laboratory Animals at the National Institute of Advanced Industrial Science and Technology (AIST), and all procedures were approved by the Animal Care and Use Committee at AIST (approval no. 2013-054).
Neuronal Cell CultureWe prepared neuronal cells from day 14 PER2::LUC mouse embryos. Cerebral cortices collected from 2- to 3-L batches of embryos were digested in phosphate buffered saline (PBS) containing 0.05% trypsin (Sigma-Aldrich, Tokyo, Japan) and 1 µg/mL of DNaseI (Gibco, Tokyo, Japan) at 37°C for 10 min. The digest was disrupted by gentle pipetting 50 to 60 times and then passed through a 40-µm cell strainer. The cells (5×105/mL) were seeded in dishes coated with poly-HEMA (Sigma) and incubated in proliferation medium (Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Gibco) containing 20 ng/mL bFGF (R&D Systems, Minneapolis, MN, U.S.A.), 20 ng/mL epidermal growth factor (EGF) (R&D Systems), B27 supplements (Gibco), 10 units/mL of penicillin and 100 µg/mL of streptomycin) at 37°C under a 5% CO2 atmosphere. Cells that formed clusters (neurospheres) were passaged every 3–4 d by centrifugation at 1200 rpm and re-seeded (5×105/mL) in fresh proliferation medium. Sub-cultured neurospheres (1×106/dish) were dissociated again into single cells in 35-mm dishes coated with poly-L-lysine (Sigma). The cells were initially incubated in proliferation medium for 24 h and cultured in differentiation medium (DMEM/F12 containing 2% fetal bovine serum, B27 and antibiotics) for 7 d to differentiate them into neuronal cells.
Semi-quantitative Reverse Transcription-Polymerase Chain Reaction (PCR) for Mouse Embryonic Fibroblasts (MEFs)Mouse embryonic fibroblasts derived from PER2::LUC mice were reached confluence as described.17) These MEFs were stimulated at time 0 h with 20 µM harmine or with 100 nM dexamethasone.18) The cells were then washed with ice-cold PBS, harvested at the indicated times in RNAiso (TaKaRa Bio, Otsu, Japan) and stored at −80°C. Total RNA isolated from the cells was assessed by real-time PCR as described.19)
Real-Time Reporter Gene Assays for MEFs and Neuronal CellsMouse embryonic fibroblasts were stimulated with 100 nM dexamethasone for 2 h, incubated in DMEM containing 0.1 mM luciferin (Wako, Tokyo, Japan), 10 mM N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES) and 10 µM harmine at 37°C, and then bioluminescence produced by PER2::LUC protein was continuously measured using the LM2400 photon detector (Hamamatsu Photonics, Hamamatsu, Japan).
Neuronal cells stimulated with 10 µM forskolin for 2 h were incubated in differentiation medium supplemented with 0.1 mM luciferin, 10 mM HEPES and 20 µM harmine at 37°C, and then the amount of bioluminescence produced by PER2::LUC protein was measured as described above. Cycloheximide (20 µg/mL) was added to neuronal cells in several dishes to determine the rate at which PER2::LUC protein decayed.
Real-Time Reporter Gene Assays of SCN SlicesMale PER2::LUC mice (8–9 weeks old) were killed by cervical dislocation at 10:00 to obtain SCN slices. Excised brains were placed in ice-cold Ringer’s solution for 10 min. Coronal slices (250 µm thick) of the hypothalamus containing the SCN were prepared in ice-cold HBSS cutting buffer (Sigma) containing 10 mM HEPES, 4.5 mM NaHCO3 and antibiotics on a DTK-1000 microslicer (Dosaka EM Co., Ltd., Osaka, Japan). The left and right halves of the SCN were trimmed to about 1 mm2 in cutting buffer and transferred to 35-mm dishes. One half was incubated at 37°C in DMEM tissue culture medium (Nissui Pharmaceutical, Tokyo, Japan) containing 10 mM HEPES, 4.5 mM NaHCO3, B27, antibiotics and 0.1 mM luciferin with 20 µM harmine, and the other was incubated under the same conditions without harmine (control). The amount of bioluminescence produced by PER2::LUC expression was continuously measured using a photon detector. Cycloheximide (20 µg/mL) was added to neuronal cells in several dishes to determine the rate at which PER2::LUC protein decayed.
RESULTS
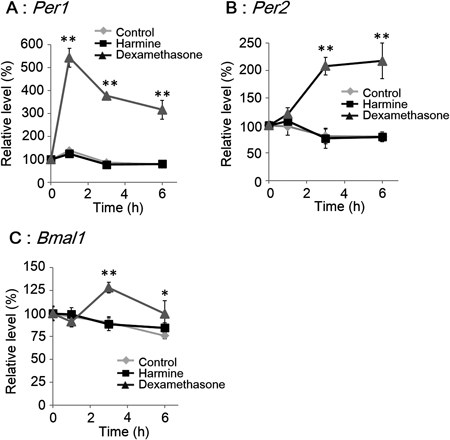
Harmine Does Not Induce Transient mRNA Expression of Clock GenesThe effects of harmine on clock gene expression were compared with those of dexamethasone to determine whether or not harmine resets the circadian clock, because dexamethasone resets the molecular circadian clock with transient expression of the clock gene, Per1.18) Harmine did not significantly affect expression levels of the clock genes, Per1, Per2 and Bmal1 (Fig. 1), whereas dexamethasone immediately increased the expression of Per1 (Fig. 1A), followed by Per2 and Bmal1 in MEFs (unpaired t-test, n=4) (Fig. 1B; C).
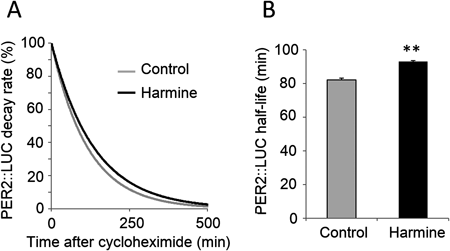
Harmine Affects the Dynamics and Decay Rate of PER2::LUC Protein in Neuronal CellsWe measured temporal expression profiles of PER2::LUC in MEFs (Fig. 2A) and in neuronal cells (Fig. 2C) derived from PER2::LUC mice to determine whether harmine affects the molecular clock in vitro. Harmine significantly lengthened the period of PER2::LUC expression by 5.99±1.20 h (unpaired t-test, n=3) in MEFs (Fig. 2B). In neuronal cells, harmine lengthened the oscillation of PER2::LUC expression by 2.95±0.48 h (unpaired t-test, n=3) in a damping manner (Figs. 2C,D), although the compound increased the amplitude for first 2 d. Dose-dependency was also found in neuronal cells (Fig. 2E) as we have previously demonstrated in NIH 3T3 fibroblasts.15) The decay rate of PER2::LUC protein was measured in neuronal cells (Fig. 3A) to determine whether harmine affects the stability of PER2 protein. Harmine significantly lengthened the half-life of PER2::LUC protein by 14.99±4.30 min (unpaired t-test, n=3) (Fig. 3B).
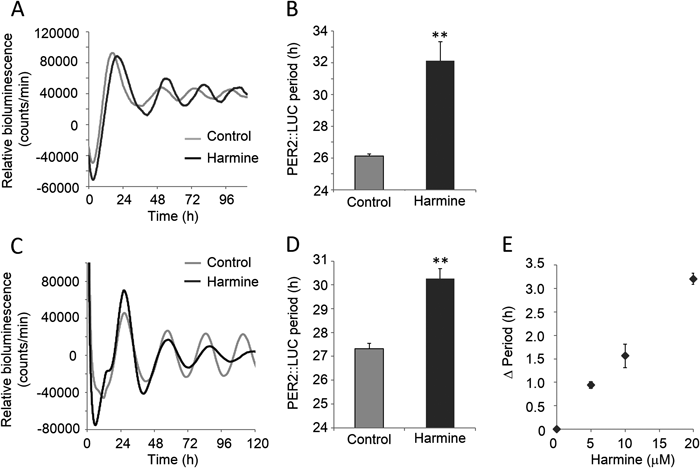
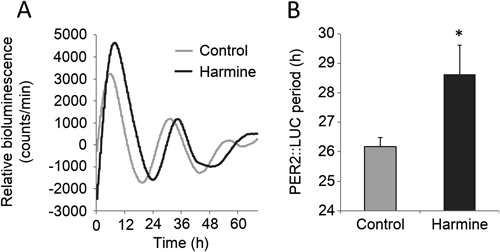
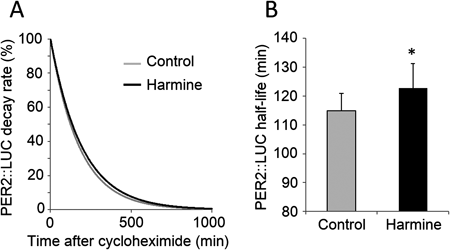
Rhythmic expression of PER2::LUC protein was measured in SCN slices to determine whether harmine affects the mammalian master clock located in the SCN (Fig. 4A). Harmine significantly lengthened the period of PER2::LUC expression by 2.45±1.04 h (paired t-test, n=6) (Fig. 4B). In addition, the decay rate of PER2::LUC protein measured in SCN slices (Fig. 5A) showed that harmine significantly lengthened the half-life of PER2::LUC protein by 7.91±2.34 min (paired t-test, n=3) (Fig. 5B).
DISCUSSION
The circadian clock primarily comprises the cell-autonomous transcriptional feedback regulation of clock genes. In addition to transcriptional regulation, the modification of core clock transcripts and proteins can also significantly affect the circadian clock in mammals.6) The present study showed that harmine lengthens the circadian period of PER2::LUC expression without the acute induction of clock genes. Harmine also extended the half-life of PER2::LUC protein both in neuronal cells and in SCN slices. Since a half-life of PER2::LUC protein is examined to reveal PER2 protein stability,20,21) harmine appears to lengthen the circadian period of the molecular clock by increasing PER2 protein stability in the SCN. The degradation of PER2 protein is intricately modulated by phosphorylation at 21 sites. Phosphorylation at Ser659 suppresses the degradation, while that at other sites encourage the degradation in mice.6) Since harmine can suppress several protein kinases,21) harmine might extend the half-life of PER2 protein with suppressing phosphorylation.
Harmine did not affect the mRNA expression of clock genes such as Per1, Per2, and Bmal1, although we previously showed that harmine induces the promoter activity of a minimal region (−197 to +27) of Bmal1 in NIH 3T3 cells.15) It is possible that the effect of harmine on the endogenous Bmal1 expression cannot be mimicked by the promoter activity of a minimal region we previously demonstrated.
Harmine has a wide spectrum of pharmacological properties, including antidepressant effects,11,12) and several psychiatric conditions including depression are closely associated with the circadian clock.14) We previously reported that harmine dose-dependently lengthens the circadian period in NIH 3T3 cells.15) However, several studies have demonstrated that the signals that are input into the circadian clock in the SCN considerably differ from those input into peripheral cells.1,2,22) Thus, how harmine affects the master clock in the SCN is critical to understand. We showed that harmine lengthens the period of PER2::LUC expression in both cultured neuronal cells and SCN slices. The lengthening effect on the circadian period in the SCN might be related to several pharmacological properties of harmine.
The lengthening effect of harmine on the circadian period of PER2::LUC expression was slightly weaker in SCN slices (2.45±1.04 h) than in cultured neuronal cells (2.95±0.48 h). In addition, chronic administration of harmine ad libitum did not affect the rhythmic expression of clock genes and circadian locomotor activity in mice (data not shown). It was reported that acute exposure to harmine does not affect spontaneous locomotor activity in the open-field test, regardless of the decreased amount of time spent in immobility and the increased amounts of time spent swimming and climbing in the forced swimming test.11) A recent study using dogs in vivo showed that harmine can diffuse into tissues (volume of distribution: 1001.53±306.08 mL/kg), where it is rapidly metabolized (half-life: 3.21±0.82 h) to harmol that is rapidly eliminated.23) Considering all of these findings together, the effects of harmine on the circadian clock might be negated weakly in the SCN ex vivo by regional regulation and strongly at the physiological level by general regulation such as that resulting from metabolism and clearance from the bloodstream. Although harmine is a potential regulator of the molecular circadian clock in the SCN, whether it can modulate the SCN function in vivo remains to be elucidated.
Acknowledgment
This project was partly supported by a Grant-in-Aid for Scientific Research (C) KAKENHI (25350179) to K. Oishi from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
REFERENCES
- 1) Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature, 418, 935–941 (2002).
- 2) Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci., 4, 649–661 (2003).
- 3) Tobler I, Borbély AA, Groos G. The effect of sleep deprivation on sleep in rats with suprachiasmatic lesions. Neurosci. Lett., 42, 49–54 (1983).
- 4) Honma S, Honma K, Shirakawa T, Hiroshige T. Rhythms in behaviors, body temperature and plasma corticosterone in SCN lesioned rats given methamphetamine. Physiol. Behav., 44, 247–255 (1988).
- 5) Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum. Mol. Genet., 15 (Spec. No. 2), R271–R277 (2006).
- 6) Vanselow K, Kramer A. Role of phosphorylation in the mammalian circadian clock. Cold Spring Harb. Symp. Quant. Biol., 72, 167–176 (2007).
- 7) Hirota T, Lee JW, Lewis WG, Zhang EE, Breton G, Liu X, Garcia M, Peters EC, Etchegaray JP, Traver D, Schultz PG, Kay SA. High-throughput chemical screen identifies a novel potent modulator of cellular circadian rhythms and reveals CKIα as a clock regulatory kinase. PLoS Biol., 8, e1000559 (2010).
- 8) Lee JW, Hirota T, Peters EC, Garcia M, Gonzalez R, Cho CY, Wu X, Schultz PG, Kay SA. A small molecule modulates circadian rhythms through phosphorylation of the period protein. Angew. Chem. Int. Ed. Engl., 50, 10608–10611 (2011).
- 9) Patel K, Gadewar M, Tripathi R, Prasad S, Patel DK. A review on medicinal importance, pharmacological activity and bioanalytical aspects of beta-carboline alkaloid “Harmine.” Asian Pac. J. Trop. Biomed., 2, 660–664 (2012).
- 10) Zheng W, Wang S, Barnes LF, Guan Y, Louis ED. Determination of harmane and harmine in human blood using reversed-phased high-performance liquid chromatography and fluorescence detection. Anal. Biochem., 279, 125–129 (2000).
- 11) Fortunato JJ, Réus GZ, Kirsch TR, Stringari RB, Stertz L, Kapczinski F, Pinto JP, Hallak JE, Zuardi AW, Crippa JA, Quevedo J. Acute harmine administration induces antidepressive-like effects and increases BDNF levels in the rat hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry, 33, 1425–1430 (2009).
- 12) Fortunato JJ, Réus GZ, Kirsch TR, Stringari RB, Fries GR, Kapczinski F, Hallak JE, Zuardi AW, Crippa JA, Quevedo J. Effects of beta-carboline harmine on behavioral and physiological parameters observed in the chronic mild stress model: further evidence of antidepressant properties. Brain Res. Bull., 81, 491–496 (2010).
- 13) Réus GZ, Stringari RB, de Souza B, Petronilho F, Dal-Pizzol F, Hallak JE, Zuardi AW, Crippa JA, Quevedo J. Harmine and imipramine promote antioxidant activities in prefrontal cortex and hippocampus. Oxid. Med. Cell. Longev., 3, 325–331 (2010).
- 14) Salvatore P, Indic P, Murray G, Baldessarini RJ. Biological rhythms and mood disorders. Dialogues Clin. Neurosci., 14, 369–379 (2012).
- 15) Onishi Y, Oishi K, Kawano Y, Yamazaki Y. The harmala alkaloid harmine is a modulator of circadian Bmal1 transcription. Biosci. Rep., 32, 45–52 (2012).
- 16) Yoo SH, Ko CH, Lowrey PL, Buhr ED, Song EJ, Chang S, Yoo OJ, Yamazaki S, Lee C, Takahashi JS. A noncanonical E-box enhancer drives mouse Period2 circadian oscillations in vivo. Proc. Natl. Acad. Sci. U.S.A., 102, 2608–2613 (2005).
- 17) Oishi K, Shirai H, Ishida N. CLOCK is involved in the circadian transactivation of peroxisome-proliferator-activated receptor alpha (PPARalpha) in mice. Biochem. J., 386, 575–581 (2005).
- 18) Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science, 289, 2344–2347 (2000).
- 19) Oishi K, Yamamoto S, Uchida D, Doi R. Ketogenic diet and fasting induce the expression of cold-inducible RNA-binding protein with time-dependent hypothermia in the mouse liver. FEBS Open Bio, 3, 192–195 (2013).
- 20) Etchegaray JP, Machida KK, Noton E, Constance CM, Dallmann R, Di Napoli MN, DeBruyne JP, Lambert CM, Yu EA, Reppert SM, Weaver DR. Casein kinase 1 delta regulates the pace of the mammalian circadian clock. Mol. Cell. Biol., 29, 3853–3866 (2009).
- 21) Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem. J., 408, 297–315 (2007).
- 22) Maywood ES, O’Neill JS, Reddy AB, Chesham JE, Prosser HM, Kyriacou CP, Godinho SI, Nolan PM, Hastings MH. Genetic and molecular analysis of the central and peripheral circadian clockwork of mice. Cold Spring Harb. Symp. Quant. Biol., 72, 85–94 (2007).
- 23) Zhang L, Teng L, Gong C, Liu W, Cheng X, Gu S, Deng Z, Wang Z, Wang C. Simultaneous determination of harmine, harmaline and their metabolites harmol and harmalol in beagle dog plasma by UPLC-ESI-MS/MS and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal., 85, 162–168 (2013).