2014 Volume 37 Issue 9 Pages 1435-1438
2014 Volume 37 Issue 9 Pages 1435-1438
Δ9-Tetrahydrocannabinol (Δ9-THC), a biologically active constituent of marijuana, possesses a wide variety of pharmacological and toxicological effects (e.g., analgesia, hypotension, reduction of inflammation, and anti-cancer effects). Among Δ9-THC’s biological activities, its recognized anti-estrogenic activity has been the subject of investigations. Since Δ9-THC is used as both a drug of abuse (marijuana) and as a preventive therapeutic to treat pain and nausea in cancer patients undergoing chemotherapy in the United States and other countries (synthesized Δ9-THC; dronabinol), it is important to investigate the mechanistic basis underlying the anti-estrogenic activity of Δ9-THC. Since Δ9-THC has “no” binding potential for estrogen receptor α (ERα) which can be activated by estrogen (E2), the question of how Δ9-THC exerts its inhibitory effect on ERα is not resolved. We have recently reported that ERβ, a second type of ER, is involved in the Δ9-THC abrogation of E2/ERα-mediated transcriptional activity. Here we discuss the possible mechanism(s) of the Δ9-THC-mediated disruption of E2/ERα signaling by presenting our recent findings as well.
Marijuana is a widespread drug of abuse that contains Δ9-tetrahydrocannabinol (Δ9-THC), a biologically active component best known for its psychotropic effects. Δ9-THC exhibits a variety of pharmacological and toxicological effects: e.g., analgesia, hypotension, reduction of inflammation, and anti-cancer effects (anti-proliferative and anti-migration effects).1–11) Among Δ9-THC’s biological activities, its recognized endocrine-disrupting effects, including anti-estrogenic activity, have been the subjects of previous investigations. It has been reported that chronic oral administration of marijuana resin is able to reduce fertility in female rats.12,13) An influence of Δ9-THC on reproductive behavior has been suspected for at least 40 years14): mechanistically in females, Δ9-THC modulates the estrous cycle and inhibits ovulation, and in males, Δ9-THC attenuates the mobility of mouse sperm.15) A Δ9-THC inhibitory effect on ovulation is also suggested in the case of the human female.12) Thus, it is possible that Δ9-THC disrupts the normal ovulatory cycle in both animals and humans.
Estrogen receptors (ERs) are hormone (17β-estradiol, E2)-dependent transcription factors existing in two forms: ERα and ERβ. ERα and ERβ have both unique and overlapping physiological roles in mediating estrogen signaling. The two forms of ERs have been known to be involved in the regulation of ovarian maturation/function and breast development.16–21) Because of the similarity in structure between Δ9-THC and E2 (i.e., phenol moiety), it was initially thought that Δ9-THC abrogation of E2/ERα signaling was attributed to the competitive inhibition of each of these toward ERα via the common moiety22); however, this early hypothesis that Δ9-THC could directly bind to the estrogen receptor has now been “abandoned” by research groups, including us.10,23–25) Furthermore, it is reported that Δ9-THC has no inhibitory activity on the CYP19A1 enzyme, also known as aromatase, which catalyzes the conversion of testosterone (an androgen) to E2 (an estrogen).26) Thus, no one can answer the question of how Δ9-THC disrupts estrogen signaling as an anti-estrogen, possibly leading to the disruption of ovarian function and breast development. Since Δ9-THC is used as both a drug of abuse (marijuana) and as a preventive therapeutic for pain and nausea in cancer patients undergoing chemotherapy in the United States and other countries (synthesized Δ9-THC; dronabinol), it is important to investigate the mechanistic basis of Δ9-THC’s E2 signaling disruption. In this study, we discuss the possible mechanism(s) of Δ9-THC-mediated disruption of E2/ERα signaling through ERβ, as revealed by our recent findings.
In general, endocrine disrupting chemicals (EDCs), including pharmaceuticals, phytoestrogens, pesticides, and industrial chemicals, can modify E2/ERα signaling via direct interaction with the ligand binding domain (LBD) of ERα via activation or inactivation (competition). ERβ, a second ER, was discovered in 1996,27) and this prompted researchers to investigate the effects of EDCs on ERs, although the physiological function is not fully understood. Some phytoestrogens can bind ERs with relatively low affinity, but display ERβ selectivity, suggesting that EDCs may have impacts on the ER subtype via specific signaling pathways. If ERβ agonism by EDCs is important to abrogate E2/ERα signaling, Δ9-THC might hijack the E2 site of ERβ. Based on this hypothesis, we investigated whether Δ9-THC could occupy the E2 binding pocket of ERβ; however, the cannabinoid did not exhibit any binding efficacy to the ERβ subtypes, at least up to 1 mM.10) Thus, it was surprising that Δ9-THC “inhibited” E2/ERα-mediated gene transcription in MCF-7 human breast cancer cells, and resulted in the inhibition of ERα-regulated cdc2 gene expression. It has been reported that Δ9-THC-induced up-regulation of lactoferrin, due to an estrogen-responsive gene in the mouse uterus, is not abrogated by a pure anti-estrogen, ICI 182,780 for ERα, and thus the authors suggest the presence of another action point(s) available for Δ9-THC action.28) The research group of Gustafsson and colleagues reported that ERβ plays a role in modulating the effects of ERα in the mouse uterus.29) At present, although we cannot explain the puzzling actions of Δ9-THC on ERα function, based on the two above-mentioned reports,28,29) we focused on the possible involvement of “ERβ” in the Δ9-THC-mediated abrogation of E2/ERα signaling (see the next Section 3).
By accumulating experimental evidence reported by many researchers, it is strongly suggested that there is yin-yang relationship between the two ERs through a mechanism underlying ERβ inhibition of ERα transcriptional activity, both in vitro and in vivo30–33): one explanation for the inhibitory effects of ERβ on ERα function is that ERβ can form functional hetero-dimers with ERα through direct protein–protein interaction coupled with the inhibition of E2/ERα signaling. In vivo studies have also shown that ERβ acts as a modulator of ERα-mediated gene transcription in the uterus.29) Because Δ9-THC has no binding potential to ERβ, if ERβ inhibition of ERα is applicable to Δ9-THC action, it would be expected that Δ9-THC positively stimulates the ERβ expression, which results in the inhibition of E2/ERα signaling. In support of our thought, it was revealed that i) Δ9-THC up-regulated ERβ mRNA/protein levels, coupled with the disruption of E2/ERα signaling underlying the suppression of genes regulated by ERα, ii) Δ9-THC-suppressed levels of ERα activity were additionally inhibited by ICI 182,780, and iii) cDNA transfection of ERβ to a Δ9-THC-treated system further accelerated the suppressive effects by Δ9-THC on ERα.10) Thus, it is suggested that ERβ is a key determinant of Δ9-THC inhibition of E2/ERα signaling. Powell and Wu demonstrated that ligand-selective induction of ERα/ERβ hetero-dimerization by means of the highly sensitive in situ bioluminescence resonance energy transfer (BRET) assay, which overcomes limitations associated with fluorescence resonance energy transfer (FRET)-based assays.33) It will be important to definitively investigate the effect of Δ9-THC as to whether it induces protein–protein association of ERα/ERβ after β-type ER up-regulation.
Some EDCs can impact ER signaling through interactions with the aryl hydrocarbon receptor (AhR), activated by a wide variety of hydrophobic ligands.34) Due to the experimental evidence that AhR signaling shares many similarities with ER signaling,35,36) there is negative cross-talk between the underlying mechanisms of ER–AhR, in which AhR impairs ER-mediated transcription through direct binding to ER target gene promoters.37) The research group of Hankinson et al. reported that Δ9-THC could induce the expression of Cyp1a1 mRNA in murine Hepa-1 cells through the AhR pathway, possibly as a ligand for AhR.38) If Δ9-THC truly acts as a ligand for AhR in our experimental systems as well, in addition to ERβ, we may need to take into consideration AhR as a modulator of ERα.
DNA methylation is a ubiquitous process of gene inactivation preferentially observed in the CpG dinucleotides. Epigenetic silencing of the ERβ gene is studied using a panel of human breast tissue samples and breast cancer cell lines, including MCF-7 and MDA-MB-231 cells, and it is suggested that ERβ expression is almost totally suppressed in breast carcinomas through the promoter DNA methylation.39–41) Based on these lines of information, we hypothesized that Δ9-THC might modulate the DNA methylation status of the ERβ gene. We first investigated the effect of 5-aza-2′-deoxycytidine (decitabine), an epigenetic modifier resulting in DNA demethylation (i.e., hypomethylation), on ERβ expression inactivated in MDA-MB-231 cells. ERβ tended to be up-regulated by decitabine in a concentration-dependent manner, and its stimulating effect was additively increased by Δ9-THC addition, implicating that Δ9-THC modulates the DNA methylation status of the ERβ gene similarly to decitabine (Takeda et al., unpublished observations).
In general, it is well known that Δ9-THC evokes its biological activities via engagement with cannabinoid receptors type 1 and type 2 (CB1 and CB2).1–11) Given that Δ9-THC utilizes both these receptors in the induction of ERβ, specific respective antagonists would be effective in inhibiting ERβ induction stimulated by the cannabinoid. Because antagonists against CB1 and CB2 receptors (SR14716A and SR144528, respectively) were not effective in the abrogation of Δ9-THC induction of ERβ in human breast cancer MDA-MB-231 cells that express the two CB receptors (Takeda et al., unpublished observations), and because Δ9-THC also induced ERβ in human breast cancer MCF-7 cells that express very low or undetectable levels of CB receptors,4,42) taken together, it is suggested that the two types of receptors are not essentially involved in the cannabinoid-mediated ERβ induction pathway(s) in breast cancer cells.
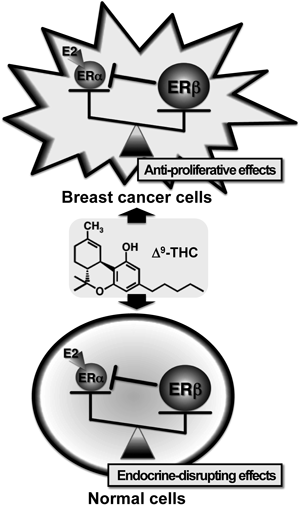
Δ9-THC may disrupt estrogen (E2) signaling by modulating the balanced expression between ERα and ERβ via up-regulation of the β type ER. If Δ9-THC could be selectively accumulated in the breast cancer cells (upper panel), its biological fate is favorable for us (i.e., anti-proliferative effects). By contrast, highly lipophilic Δ9-THC could be also distributed in normal cells (lower panel), which leads to disruption of the physiological function of E2 (i.e., endocrine disruption).
Although the benefits of Δ9-THC are apparent as an adjuvant in the treatment of cancer-related side effects during cancer chemotherapy, the recent results reported here also suggest that the cannabinoid has endocrine-disrupting potential through the possible up-regulation of ERβ (Fig. 1). Δ9-THC may have inhibitory effects on breast cancer cell proliferation by means of up-regulation of ERβ (possibly through the formation of inhibitory ERα/ERβ dimers) if Δ9-THC is selectively accumulated in cancer cells (Fig. 1; upper panel). On the other hand, at the same time, Δ9-THC may disrupt the balanced relationship between ERα and ERβ via up-regulation of the β type ER in normal cells, since Δ9-THC can be accumulated up to 20-fold in some tissues (i.e., fat tissue) due to its highly lipophilic nature.43) In addition to the recreational use of marijuana, the clinical use of Δ9-THC (dronabinol) may give rise to adverse effects on the endocrine system (Fig. 1; lower panel). We suggest that Δ9-THC may be categorized as an EDC, but clearly, further studies coupled with in vivo experiments are needed to validate our hypothesis presented here.
This study was supported in part by a Grant-in-Aid for Scientific Research (C) [25460182, (to S.T.)] from the Japan Society for the Promotion of Science (JSPS) KAKENHI. The author wishes to thank Prof. Kazuhito Watanabe, Department of Hygienic Chemistry, Faculty of Pharmaceutical Sciences, Hokuriku University, for his contributions (excellent advice and donation of purified Δ9-THC) to the studies, and students (Ms. Mari Harada, Mr. Shunsuke Okajima, Ms. Hiroko Miyoshi, Mr. Kazutaka Yoshida, Mr. Hajime Nishimura, and Mr. Yasushi Yoshioka) from the Department of Molecular Biology, Daiichi University of Pharmacy for their technical contributions to the studies.