Abstract
Berberine, an isoquinoline alkaloid derived from many medicinal plants, has been extensively used to treat various gastrointestinal diseases. In the present study, we investigated whether berberine could ameliorate intestinal mucosal barrier damage induced by peritoneal air exposure for 3 h. Peritoneal air-exposure rats received 100, 150, and 200 mg/kg berberine orally via gavage four times before and after surgery. Blood and terminal ileum samples were collected 24 h after surgery. The serum D-lactate levels were determined using an enzyme-linked immunosorbent assay (ELISA) kit. Intestinal permeability was determined by measuring the intestinal clearance of fluorescein isothiocyanate (FITC)–dextran (FD4). Intestinal inflammation was assessed by measuring myeloperoxidase activity. Intestinal histopathology was also assessed. The results revealed that berberine decreased the serum D-lactate level, intestinal FD4 clearance, and myeloperoxidase activity. Edema and inflammation were reduced by berberine in the intestinal mucosa and submucosa, and the Chiu’s scores, indices of intestinal mucosal injury, also decreased in the berberine-treated group. In addition, berberine exerted these protective effects in a dose-dependent manner, with a significant difference from the control group at doses of 150 and 200 mg/kg. The results suggest that berberine could ameliorate intestinal mucosal barrier damage induced by peritoneal air exposure, which is linked to its anti-inflammatory activity. Berberine may be a promising treatment for intestinal mucosal barrier damage in open abdominal surgery.
Berberine (BBR) is an isoquinoline alkaloid derived from many medicinal plants, such as Cortex phellodendri (Huangbai), Hydrastis canadensis (goldenseal), and Rhizoma coptidis (Huanglian).1) It has been well accepted as an oral drug to treat many gastrointestinal diseases for centuries in Chinese traditional medicine.2) Nowdays, BBR has been worldwide used as a popular traditional herbal medicine, especially in China and other Asian countries.3) During the last few decades, many studies have shown that BBR exhibits multiple pharmacological activities including anti-oxidant, anti-microbial, anti-tumor, and cholesterol-lowering effects.4–7) Our previous studies also found that BBR could reinforce the intestinal epithelial tight junction and reduce epithelial intestinal permeability in vitro and in vivo, which is important to maintain the intestinal mucosal barrier function.8–10)
Peritoneal air exposure is a common clinical phenomenon in open abdominal surgery, but could induce injury to various tissues/organs.11–13) The intestinal tract is one of the target organs during various stress.14) Our recent studies found that peritoneal air exposure could induce damage to the intestinal mucosal barrier, which is proportional to the time length of peritoneal air exposure.15) It is well known that damage to the intestinal mucosa barrier will result in intestinal bacterial and endotoxin translocation and further contribute to local and systemic inflammation.16) The injury and inflammation in the intestinal tract is often considered as the driving force of many complications after open abdominal surgery.17–19) Therefore, methods to explore effective treatment for intestinal mucosal barrier damage are popular pursuits in modern surgery.
In the present study, therefore, we designed to investigate whether BBR could ameliorate intestinal mucosal barrier damage induced by peritoneal air exposure under experimental conditions mimicking clinical operation room atmosphere.
MATERIALS AND METHODS
AnimalsHealthy adult male Sprague-Dawley rats (weighing 210 to 230 g) were obtained from Jinling Hospital, Nanjing, China. The rats were housed in our laboratory in a temperature- and humidity-controlled environment; they had free access to standard rat chow and tap water. The lights were maintained on a 12 : 12-h light : dark cycle. This animal use and care protocol and experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Jinling Hospital. The experiments were also performed according to the National Institutes of Health Guidelines on the use of laboratory animals.
DrugsBBR chloride was purchased from Sigma-Aldrich, St. Louis, MO, U.S.A. All chemicals and reagents were procured from local suppliers and were of analytical grade.
Animal Grouping and AdministrationAfter an adaptation period for one week, 30 rats were randomly divided into 5 groups (n=6 each): a control group (CG), a exposure group with peritoneal air exposure for 3 h (EG), and three BBR-treated EG groups at a dose of 100, 150, and 200 mg/kg, respectively (BG, including BG100, BG150, and BG200). The peritoneal air exposure was induced as described in our previous study.15) Briefly, after full anesthesia with 2% pentobarbital sodium (3.5 mL/kg), rats in the EG and the BG groups were laparotomized through a 3 cm midline abdominal incision, and then the wound edge was retracted to allow for maximal peritoneal air exposure for 3 h. For the CG group, the animals underwent the same anesthesia but without any operative procedures. The surgical procedures were performed in an aseptic environment with controlled temperature and humidity.
Rats in three BG groups were separately administrated BBR orally at a dose of 100, 150, and 200 mg/kg through gavage once daily at 3 d, 2 d, and 1 d before surgery, and at 6 h after surgery.8,20,21) Each dose was dissolved in saline and diluted to a final concentration of 10 mg/mL, and the CG and the EG groups received an equal volume of saline four times by the same method as the BG groups. At 24 h after surgery, samples of blood and terminal ileum were obtained for the further analysis. The selection of terminal ileum as the investigation site was based on previous studies.22–24) These studies have indicated that the terminal ileum is the major site for observation of damage to intestinal mucosal barrier functions following exposure to various injuries. It is suggested that terminal ileum is the most sensitive section of the intestinal tract.25)
D-Lactate (D-LA) DeterminationThe blood samples were obtained from the inferior vena cava. The serum was prepared by centrifugation at a speed of 1500 rpm for 15 min at 4°C, and the levels of D-LA were determined with an ELISA kit for rats (R&D Systems, Germany) according to the manufacturer’s instructions.
Intestinal Permeability DeterminationThe intestinal permeability was determined by calculating intestinal clearance of fluorescein isothiocyanate (FITC) dextran (FD4) as described in our previous studies.15,25) Briefly, a segment of terminal ileum with length of 8 cm was prepared, and the mucosa was everted. One end of the intestinal segment was ligated, and a gut sac was made by injecting 1.0 mL of Krebs–Henseleit bicarbonate buffer from the other end. The sac was then suspended in a solution containing 0.5 mg/mL of FD4 (average molecular weight: 4000), and the temperature was maintained at 37°C. The bathing solution was aerated by gently bubbling with a gas mixture containing 5% CO2 and 95% O2. Thirty minutes later, the value of the mucosal surface area (A) was calculated. The fluorescence of the solution was measured by a fluorescence spectrophotometer and the clearance of FD4 was calculated according to the following formula:
Where
C is the mucosal to serosal clearance of FD4 in µL·min
−1·cm
−2; [FD4]
ser is the FD4 concentration in the serosal fluid aspirated from the sac at the end of the 30 min period; [FD4]
muc is the FD4 concentration in the mucosal fluid aspirated from the sac at the begining of the 30 min period;
L is the length of the sac and
D is the diameter of the sac.
Intestinal Myeloperoxidase (MPO) Activity AssessmentThe ileum tissue was homogenized, centrifuged at a speed of 2000×g, at 4°C for 15 min, and then the supernatants were obtained. The protein concentration in the supernatant was determined using the method of Bradford. MPO activity was quantitatively measured by spectrophotometry at 460 nm as described in our previous study.26) Values were expressed as units/g in the ileum tissue.
HistopathologyThe terminal ileum was fixed in 4% buffered formaldehyde, and embedded in paraffin. Slices of 4-µm thick were prepared, stained with hematoxylin and eosin (H&E), and then examined by a pathologist blinded to this study design using light microscopy. The degree of intestinal mucosa injury was assessed by using Chiu’s scoring system as described previously.27) The intestinal mucosal changes after peritoneal air exposure graded as follows: Grade 0, Normal mucosal villi; Grade 1, Development of sub-epithelial Gruenhagen’s space, usually seen at the apex of the villus; often with capillary congestion; Grade 2, Extension of the sub-epithelial Gruenhagen’s space with moderate lifting of epithelial layer from the lamina propria; Grade 3, Massive epithelial lifting down the sides of villi, with a few tips being denuded; Grade 4, Denuded villi with lamina propria and dilated capillaries exposed; Increased cellularity of lamina propria; and Grade 5, Digestion and disintegration of lamina propria; hemorrhage and ulceration. The average scores of the exposure groups were compared with that of the controls.
Statistical AnalysisData were expressed as the mean±S.D. Statistical analyses were performed using SPSS 17.0 software (SPSS Inc., U.S.A.). Data were analyzed using one-way ANOVA after homogeneity test for variance. Significant results were then analyzed post hoc using the Dunnett t-test. The correlation of two variables was performed using Pearson correlation analysis. Differences were considered statistically significant when p<0.05.
RESULTS
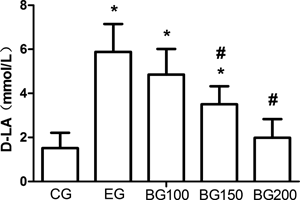
Effects of BBR on D-LA LevelThe results were shown in Fig. 1. The D-LA level of the EG group was significantly increased (p<0.05) when compared with that of the CG group. However, BBR induced a progressive decrease in the D-LA level in a dose-dependent manner, differed significantly in the BG150 and the BG200 groups when compared with that in the EG group (p<0.05). In addition, while the D-LA level of the BG100 and the BG150 groups was still significantly higher than that of the CG group (p<0.05), that of the BG200 group returned to the level of the CG group.
Effects of BBR on Intestinal PermeabilityThe results were shown in Fig. 2. The intestinal clearance of FD4 of the EG group were significantly increased (p<0.05) when compared with that of the CG group. However, BBR induced a progressive decrease in the intestinal clearance of FD4 in a dose-dependent manner differed significantly in the BG150 and the BG200 groups when compared with that in the EG group (p<0.05). In addition, while the intestinal clearance of FD4 of the BG100 and the BG150 groups was still significantly higher than that of the CG group (p<0.05), that of the BG200 group returned to the level of the CG group.
Effects of BBR on Intestinal MPO ActivityThe results were shown in Fig. 3. The MPO activity of the EG group was significantly increased (p<0.05) when compared with that of the CG group. However, BBR induced a progressive decrease in the MPO activity in a dose-dependent manner, differed significantly in the BG150 and the BG200 groups when compared with that in the EG group (p<0.05). In addition, while the MPO activity of the BG100 group was still significantly higher than that of the CG group (p<0.05), that of the BG150 and the BG200 groups returned to the level of the CG group.
HistopathologyThe results were shown in Fig. 4. There was no obvious structural injury in intestinal tissue among groups. However, edema and inflammation were observed in the intestinal mucosa and submucosa in the EG group when compared with that of the CG group, while BBR reduced this edema and in three BG groups when compared with that of the EG group. In addition, as shown in Fig. 5, BBR also caused a progressive decrease in Chiu’s score, differed significantly in the BG150 and the BG200 groups, when compared with the EG group (p<0.05).
DISCUSSION
In the present study, we designed to investigate whether BBR could ameliorate intestinal mucosal barrier damage induced by peritoneal air exposure under experimental conditions mimicking clinical operation room atmosphere. Our results showed that BBR decreased serum D-lactate level, intestinal FD4 clearance, and intestinal MPO activity. The edema and inflammation was reduced by BBR in intestinal mucosa and submucosa, and the Chiu’s scores, indices for intestinal mucosal injury, was also decreased in the BBR treated group. In addition, BBR exerted this protective effect in a dose-dependent manner, and with significance at doses of 150 and 200 mg/kg.
Peritoneal air exposure is a common clinical phenomenon in open abdominal surgery, but could induce injury to various tissues/organs.11–13) Gut is considered as one of the target organs and highly sensitive to suffer from injury during various stress.14,28,29) Our recent studies found that peritoneal air exposure could induce damage to the intestinal mucosal barrier, which is proportional to the time length of peritoneal air exposure.15) As we known, bacterial colonization of the gut is extensive and bacteria are confined to the gastrointestinal tract by the intestinal mucosal barrier. Damage to the intestinal mucosa barrier will result in intestinal bacteria and endotoxin translocation, and further contribute to local and systemic inflammation and other complications.16,30,31)
BBR is a traditional herbal used to treat many gastrointestinal diseases worldwide.2,3) Many studies have shown that BBR exhibits multiple pharmacological activities including anti-oxidant, anti-microbial, anti-tumor, and cholesterol-lowering effects.4–7) Our previous studies found that BBR could reinforce the intestinal epithelial tight junction and reduce epithelial intestinal permeability in vitro and in vivo, which is important to maintain the intestinal mucosal barrier function.8–10) Therefore, in order to further explore the protective effect of BBR on the intestinal mucosal barrier damage induced by peritoneal air exposure, it is important to determinate the changes in the intestinal mucosa barrier function during peritoneal air exposure under experimental conditions mimicking clinical operation room atmosphere.
Nowadays, it is a challenging task to measure the intestinal mucosal barrier function. In many published studies, measurement of the intestinal permeability is used to indirectly assess the intestinal mucosal barrier function. For example, D-lactate has been employed as a biomarker for the intestinal permeability, especially for bacterial infection.32–35) Therefore, we choose D-lactate as a marker for the changes in intestinal mucosal barrier function. In addition, FD4 is a relatively large molecule, which could not come across the normal intestinal mucosal barrier. When the intestinal permeability is increased under pathological conditions, FD4 can penetrate the intestinal mucosal barrier. Therefore, the FD4 clearance is also employed as a good marker for intestinal mucosal barrier function.25,36) In the present study, we found that BBR caused decreases in both serum D-lactate level and intestinal clearance of FD4. In addition, although there was no significant changes in histopathology among groups, the Chiu’s scores assessed for intestinal mucosal injury were significantly decreased by BBR when compared with the peritoneal air exposure group. In addition, BBR exerted this protective effect in a dose-dependent manner, and with significance at doses of 150 and 200 mg/kg. Therefore, all of these results indicated that BBR could ameliorate intestinal mucosal barrier damage induced by peritoneal air exposure. Based on these results, in clinical practice, when patients undergo excessive abdominal surgery, especially open abdominal surgery, adminstration of BBR perioperatively may be a promising treatment to ameliorate intestinal mucosal barrier damage, decrease systemic inflammatory response, and further enhance recovery after surgery.
However, when we designed the current experiments, considering that BBR has multiple pharmacological activities including anti-inflammation, anti-microbiotic action, and other organ function protection,2,4–7) we were not sure which pharmacological activity BBR exerted to improve the protective effect on the intestinal mucosal barrier damage induced by peritoneal air exposure. Since many papers have shown that intestinal inflammation is critical in many gastrointestinal diseases, and reducing intestinal inflammation may improve various intestinal injury,37–39) we therefore determined the inflammation factor, i.e., MPO activity, in the intestine, but did not investigate the anti-microbiotic action and other pharmacological activities of BBR. The results showed that the intestinal MPO activity, a sensitive index to evaluate inflammation process, was increased by peritoneal air exposure for three hours, which was consistent with our previous study.13) However, BBR could decrease the intestinal MPO activity in a dose-dependent manner, differed significantly at doses of 150 and 200 mg/kg. These results showed that the protective effect of BBR on the intestinal mucosal barrier damage induced by peritoneal air exposure may be related to the anti-inflammatory activity. Nevertheless, the further study emphasizing the anti-microbiotic action and other pharmacological activities of BBR is underway to explore the exact mechanism of BBR’s protective effect on intestinal barrier function after peritoneal air exposure.
In conclusion, our data demonstrates that BBR could ameliorate intestinal mucosal barrier damage induced by peritoneal air exposure, which is linked to the anti-inflammatory activity. BBR may be a promising treatment for intestinal mucosal barrier damage in open abdominal surgery.
Acknowledgment
The authors would like to thank Prof. Qiurong Li, Research Institute of General Surgery at Jinling Hospital, for her excellent technical assistance. This study was supported by 12th five-year Major Program of Army Grants (AWS12J001); Jiangsu Province’s Special Project of Science and Technology in Medicine (BL2012006).
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- 1) Wang Z, Chen Z, Yang S, Wang Y, Huang Z, Gao J, Tu S, Rao Z. Berberine ameliorates collagen-induced arthritis in rats associated with anti-inflammatory and anti-angiogenic effects. Inflammation, 37, 1789–1798 (2014).
- 2) Chen C, Yu Z, Li Y, Fichna J, Storr M. Effects of berberine in the gastrointestinal tract—A review of actions and therapeutic implications. Am. J. Chin. Med., 42, 1053–1070 (2014).
- 3) Chen K, Li G, Geng F, Zhang Z, Li J, Yang M, Dong L, Gao F. Berberine reduces ischemia/reperfusion-induced myocardial apoptosis via activating AMPK and PI3K-Akt signaling in diabetic rats. Apoptosis, 19, 946–957 (2014).
- 4) Hur JM, Kim D. Berberine inhibited radioresistant effects and enhanced anti-tumor effects in the irradiated-human prostate cancer cells. Toxicol. Res., 26, 109–115 (2010).
- 5) Li YH, Yang P, Kong WJ, Wang YX, Hu CQ, Zuo ZY, Wang YM, Gao H, Gao LM, Feng YC, Du NN, Liu Y, Song DQ, Jiang JD. Berberine analogues as a novel class of the low-density-lipoprotein receptor up-regulators: synthesis, structure–activity relationships, and cholesterol-lowering efficacy. J. Med. Chem., 52, 492–501 (2009).
- 6) Zhang BJ, Xu D, Guo Y, Ping J, Chen LB, Wang H. Protection by and anti-oxidant mechanism of berberine against rat liver fibrosis induced by multiple hepatotoxic factors. Clin. Exp. Pharmacol. Physiol., 35, 303–309 (2008).
- 7) Marinova EK, Nikolova DB, Popova DN, Gallacher GB, Ivanovska ND. Suppression of experimental autoimmune tubulointerstitial nephritis in BALB/c mice by berberine. Immunopharmacology, 48, 9–16 (2000).
- 8) Gu L, Li N, Gong J, Li Q, Zhu W, Li J. Berberine ameliorates intestinal epithelial tight-junction damage and down-regulates myosin light chain kinase pathways in a mouse model of endotoxinemia. J. Infect. Dis., 203, 1602–1612 (2011).
- 9) Li N, Gu L, Qu L, Gong J, Li Q, Zhu W, Li J. Berberine attenuates pro-inflammatory cytokine-induced tight junction disruption in an in vitro model of intestinal epithelial cells. Eur. J. Pharm. Sci., 40, 1–8 (2010).
- 10) Gu L, Li N, Li Q, Zhang Q, Wang C, Zhu W, Li J. The effect of berberine in vitro on tight junctions in human Caco-2 intestinal epithelial cells. Fitoterapia, 80, 241–248 (2009).
- 11) Southall JC, Lee SW, Bessler M, Allendorf JD, Whelan RL. The effect of peritoneal air exposure on postoperative tumor growth. Surg. Endosc., 12, 348–350 (1998).
- 12) Watson RW, Redmond HP, McCarthy J, Burke PE, Bouchier-Hayes D. Exposure of the peritoneal cavity to air regulates early inflammatory responses to surgery in a murine model. Br. J. Surg., 82, 1060–1065 (1995).
- 13) Tan S, Yu W, Lin Z, Chen Q, Shi J, Dong Y, Duan K, Bai X, Xu L, Li J, Li N. Peritoneal air exposure elicits an intestinal inflammation resulting in postoperative ileus. Mediators Inflamm., 2014, 924296 (2014).
- 14) Gatt M, Reddy BS, MacFie J. Review article: bacterial translocation in the critically ill—evidence and methods of prevention. Aliment. Pharmacol. Ther., 25, 741–757 (2007).
- 15) Bao J, Tan S, Yu W, Lin Z, Dong Y, Chen Q, Shi J, Duan K, Bai X, Xu L, Li J, Li N. The effect of peritoneal air exposure on intestinal mucosal barrier. Gastroenterol. Res. Pract., 2014, 674875 (2014).
- 16) Costa KA, Soares ADN, Wanner SP, Santos RGC, Fernandes SOA, Martins FS, Nicoli JR, Coimbra CC, Cardoso VN. L-Arginine supplementation prevents increases in intestinal permeability and bacterial translocation in male Swiss mice subjected to physical exercise under environmental heat stress. J. Nutr., 144, 218–223 (2014).
- 17) Costes LM, van der Vliet J, van Bree SH, Boeckxstaens GE, Cailotto C. Endogenous vagal activation dampens intestinal inflammation independently of splenic innervation in postoperative ileus. Auton. Neurosci., 185, 76–82 (2014).
- 18) The FO, Bennink RJ, Ankum WM, Buist MR, Busch OR, Gouma DJ, van der Heide S, van den Wijngaard RM, de Jonge WJ, Boeckxstaens GE. Intestinal handling-induced mast cell activation and inflammation in human postoperative ileus. Gut, 57, 33–40 (2008).
- 19) Wang P, Wei X, Li Y, Li J. Influences of intestinal ligation on bacterial translocation and inflammatory response in rats with hemorrhagic shock: implications for damage control surgery. J. Invest. Surg., 21, 244–254 (2008).
- 20) Xing LJ, Zhang L, Liu T, Hua YQ, Zheng PY, Ji G. Berberine reducing insulin resistance by up-regulating IRS-2 mRNA expression in nonalcoholic fatty liver disease (NAFLD) rat liver. Eur. J. Pharmacol., 668, 467–471 (2011).
- 21) Endo M, Hori M, Ozaki H, Oikawa T, Hanawa T. Daikenchuto, a traditional Japanese herbal medicine, ameliorates postoperative ileus by anti-inflammatory action through nicotinic acetylcholine receptors. J. Gastroenterol., 49, 1026–1039 (2014).
- 22) Wu J, Chen J, Wu W, Shi J, Zhong Y, van Tol EA, Tang Q, Cai W. Enteral supplementation of bovine lactoferrin improves gut barrier function in rats after massive bowel resection. Br. J. Nutr., 112, 486–492 (2014).
- 23) Son JY, Chandler B, Feketova E, Qin Y, Quackenbush EJ, Deitch EA. Oral pretreatment with recombinant human lactoferrin limits trauma-hemorrhagic shock-induced gut injury and the biological activity of mesenteric lymph. J. Surg. Res., 187, 270–277 (2014).
- 24) Chang R, Wang Y, Chang J, Wen L, Jiang Z, Yang T, Yu K. LPS preconditioning ameliorates intestinal injury in a rat model of hemorrhagic shock. Inflamm. Res., 63, 675–682 (2014).
- 25) Yue C, Wang W, Tian WL, Huang Q, Zhao RS, Zhao YZ, Li QR, Li JS. Lipopolysaccharide-induced failure of the gut barrier is site-specific and inhibitable by growth hormone. Inflamm. Res., 62, 407–415 (2013).
- 26) Tan S, Yu W, Lin Z, Chen Q, Shi J, Dong Y, Duan K, Bai X, Xu L, Li J, Li N. Anti-inflammatory effect of ginsenoside Rb1 contributes to the recovery of gastrointestinal motility in the rat model of postoperative ileus. Biol. Pharm. Bull., 37, 1788–1794 (2014).
- 27) Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch. Surg., 101, 478–483 (1970).
- 28) Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia–reperfusion injury of the intestine and protective strategies against injury. Dig. Dis. Sci., 49, 1359–1377 (2004).
- 29) Gu L, Li N, Yu W, Gong J, Li Q, Zhu W, Li J. Berberine reduces rat intestinal tight junction injury induced by ischemia–reperfusion associated with the suppression of inducible nitric oxide synthesis. Am. J. Chin. Med., 41, 1297–1312 (2013).
- 30) Tian R, Tan JT, Wang RL, Xie H, Qian YB, Yu KL. The role of intestinal mucosa oxidative stress in gut barrier dysfunction of severe acute pancreatitis. Eur. Rev. Med. Pharmacol. Sci., 17, 349–355 (2013).
- 31) Zhang X, Jiang X. Effects of enteral nutrition on the barrier function of the intestinal mucosa and dopamine receptor expression in rats with traumatic brain injury. JPEN J. Parenter. Enteral Nutr., 2013, 24047867 (2013).
- 32) Wang H, Gong J, Wang W, Long Y, Fu X, Fu Y, Qian W, Hou X. Are there any different effects of Bifidobacterium, Lactobacillus and Streptococcus on intestinal sensation, barrier function and intestinal immunity in PI-IBS mouse model? PLoS ONE, 9, e90153 (2014).
- 33) Liang HY, Chen T, Wang T, Huang Z, Yan HT, Tang LJ. Time course of intestinal barrier function injury in a sodium taurocholate-induced severe acute pancreatitis in rat model. J. Dig. Dis., 15, 386–393 (2014).
- 34) Sun YJ, Song DD, Diao YG, Zhou J, Zhang TZ. Penehyclidine hydrochloride preserves the intestinal barrier function in patients undergoing cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg., 146, 179–185 (2013).
- 35) Li WD, Jia L, Ou Y, Huang YX, Jiang SM. Surveillance of intra-abdominal pressure and intestinal barrier function in a rat model of acute necrotizing pancreatitis and its potential early therapeutic window. PLoS ONE, 8, e78975 (2013).
- 36) Wattanasirichaigoon S, Menconi MJ, Delude RL, Fink MP. Effect of mesenteric ischemia and reperfusion or hemorrhagic shock on intestinal mucosal permeability and ATP content in rats. Shock, 12, 127–133 (1999).
- 37) Rychter J, Clave P. Intestinal inflammation in postoperative ileus: pathogenesis and therapeutic targets. Gut, 62, 1534–1535 (2013).
- 38) Karhausen J, Qing M, Gibson A, Moeser AJ, Griefingholt H, Hale LP, Abraham SN, Mackensen GB. Intestinal mast cells mediate gut injury and systemic inflammation in a rat model of deep hypothermic circulatory arrest. Crit. Care Med., 41, e200–e210 (2013).
- 39) Costantini TW, Peterson CY, Kroll L, Loomis WH, Putnam JG, Wolf P, Eliceiri BP, Baird A, Bansal V, Coimbra R. Burns, inflammation, and intestinal injury: protective effects of an anti-inflammatory resuscitation strategy. J. Trauma, 67, 1162–1168 (2009).