2015 Volume 38 Issue 10 Pages 1439-1449
2015 Volume 38 Issue 10 Pages 1439-1449
Adult hippocampal neurogenesis occurs in the dentate gyrus (DG) of the mouse hippocampus, and plays roles in learning and memory progresses. In amyloid precursor protein (APP)/presenilin 1 (PS1) transgenic mice, a rodent model of Alzheimer’s disease (AD), severe impairment of neurogenesis in the dentate subgranular zone (SGZ) of the DG has been reported. Osthole, an active constituent of Cnidium monnieri (L.) CUSSON, has been reported to exert neuroprotective effects and may promote neural stem cell proliferation. However, whether osthole ameliorates spatial memory deficits and improves hippocampal neurogenesis in APP/PS1 mice remains unknown. In this study we found that osthole (30 mg/kg intraperitoneally (i.p.) once daily) treatment dramatically ameliorated the cognitive impairments by Morris Water Maze test and passive avoidance test, and augmented neurogenesis in the DG of hippocampus in APP/PS1 mice. Furthermore, osthole treatment upregulated expression of brain-derived neurotrophic factor (BDNF) and enhanced activation of the BDNF receptor tyrosine receptor kinase B (TrkB) following increased phosphorylation of cyclic AMP response element-binding protein (CREB), indicating that osthole improves neurogenesis via stimulating BDNF/TrkB/CREB signaling in APP/PS1 transgenic mice.
Alzheimer’s disease (AD), the most common form of dementia, is a neurodegeneration disease with the hallmarks of aggregation of amyloid-β (Aβ) peptides outside the neurons and neurofibrillary tangles (NFTs) intra neurons.1–3) These pathological features result in neural loss and brain atrophy as the disease progresses,3,4) and cognitive dysfunction such as spatial learning & memory deficits.2,5,6)
It has been shown that neurogenesis occurs in the adult mammalian brain and plays roles in both learning & memory progresses and recovery from injury.7,8) There are two sites of adult neurogenesis in the rodent central nervous system (CNS): the cortical subventricular zone (SVZ) and the dentate subgranular zone (SGZ) in the hippocampal formation. In the dentate gyrus (DG), adult neurogenesis refines network functions by constant addition of new neurons to the granule cell layer (GCL).9–11) Adult hippocampal neurogenesis is a unique form of neural circuit plasticity that results in the generation of new neurons throughout life. Neurons that arise in adults (adult-born neurons) show heightened synaptic plasticity during their maturation and can account for up to ten percent of the entire granule cell population.12) However, many AD risk factors which are associated with cognitive impairments affect hippocampal neurogenesis.7,8,13) In models of AD such as mice with mutation in amyloid precursor protein (APP) or presenilin 1 (PS1), severe impairment of neurogenesis in the SGZ of the DG has been reported.13,14) In the hippocampus of patients with AD, a compensatory enhancement of neurogenesis has been observed, but this enhanced neurogenesis is not able to compensate for severe neuronal loss.15) All these findings suggest that impaired neurogenesis may attribute to the pathogenesis of AD. Therefore, augmentation of hippocampal neurogenesis have therapeutic potential to the treatment of AD.
Brain-derived neurotrophic factor (BDNF) and its receptor, tyrosine receptor kinase B (TrkB), are broadly expressed in the adult mammalian brain. The neurotrophic action of BDNF is mediated primarily by binding with its high affinity receptor, TrkB, which leads to receptor dimerization and phosphorylation of multiple tyrosine residues.16) BDNF/TrkB-stimulated intracellular signaling is critical for hippocampal neurogenesis.17–19) The activation of BDNF/TrkB signaling lead to the phosphorylation of cyclic AMP response element-binding protein (CREB),20) which has been characterized to be a critical process for the transcriptional regulation of hippocampal neurogenesis. Thus, the strategy to stimulate BDNF/TrkB/CREB signaling exert beneficial effects on neurogenesis.
Osthole (7-methoxy-8-isopentenoxycoumarin, C15H16O3, molecular weight (MW)=244.39), a natural coumarin derivative isolated from many medicinal plants such as Cnidium monnieri (L.) CUSSON, has taken considerable attention because of its diverse pharmacological functions and is considered to have potential therapeutic applications on the treatment of AD.21) It has been previously reported that osthole exerts neuroprotective effects in some experimental models of cerebral ischemia/reperfusion and brain injury via anti-oxidative and anti-inflammatory activities,22–26) and it attenuates clinical severity and demyelination of experimental autoimmune encephalomyelitis (EAE) in mice.27) Recently osthole has been reported to be capable of improving the proliferation of neural stem cells (NSCs) in vitro and enhancing the expression of BDNF in the CNS,28) displaying a promising capacity to augment adult hippocampal neurogenesis in AD.
Therefore, the present study investigated whether osthole treatment improves cognitive function and increased the adult neurogenesis of hippocampus in APP/PS1 transgenic mice, a rodent model of AD. We treated APP/PS1 mice with osthole for 4 weeks, and examined the attenuation of spatial learning & memory deficits. Furthermore, the possible therapeutic mechanisms of augmentation of hippocampal neurogenesis and upregulation of BDNF/TrkB/CREB signaling were investigated.
Osthole was purchased from Sigma-Aldrich Co. (Catalog number: O9265; Molecular formula: C15H16O3; MW=244.29) and kept protecting from light during the experiments. Stock solution of osthole (1 mM) was dissolved in phosphate buffered saline (PBS) and stored at −20°C.
AnimalsAPP/PS1 transgenic mice (male, age 8–9 months) were obtained from the Model Animal Research Center of Nanjing University. These mice express a chimerical mouse/human APP containing the K595N/M596L Swedish mutations and a mutant human PS1 carrying the exon 9-deleted variant under the control of mouse prion promoter elements, directing transgene expression predominantly to CNS neurons.29,30) The wild-type (WT) C57BL/6 mice (male, age 8–9 months) were used as control compared with APP/PS1 transgenic mice.
All animal protocols were approved by the Animal Care Commitee in TaiShan Medical University (Taian, China) and performed according to institutional guidelines. All mice were housed in cages in a controlled environment (22–25°C, 55% relative humidity, 12 h light/dark cycle) with free access to food and water, and maintained in a specific pathogen-free environment.
Experimental Animal GroupsFor behavioral test, mice were randomly divided into 4 groups: (1) WT control mice, each mouse was administered saline via intraperitoneal injections (i.p.) as the volume of osthole treatment group; (2) APP/PS1 transgenic mice, every mouse received saline via i.p. as the same volume of control group; (3) Osthole-treated APP/PS1 mice and (4) Osthole-treated WT mice, every animal was administered osthole via i.p. at a dosage of 30 mg/kg body weight once a day according to previous reports,22,28) this therapy was last for 28 d; n=15 for each group. For paraffin sections and immunohistochemistry analysis of Aβ(1–40), n=6 animals from groups. For 5-bromo-2′-deoxyuridine (BrdU) incorporation and immunohistochemical studies, n=6 animals from groups. For Western blot analysis, n=3 mice for each group.
Passive Avoidance TestAfter 4 weeks of the osthole treatment, the learning-memory ability of mice was evaluated by the passive avoidance test (PAT), as previously described.31,32) Passive avoidance apparatus for training and testing contained a box of two compartments, one light (white compartment) and the other dark (dark compartment) with the same size connected with an automatic guillotine door. The dark compartment is equipped with a grid floor placed through which a foot-shock can be delivered. In brief, we acquired 3 min adaptation and aversion trials in the apparatus during the training sessions. The test session was performed 24 h after the training session. In the test session, each mouse was placed in the light compartment and allowed to explore 3 min, and then the guillotine door was raised. The latencies and frequencies for mice to enter the dark compartment were recorded during the whole testing period (5 min).
Morris Water MazeThe conventional hidden platform version of the Morris water maze test was used to test spatial memory in mice. The Morris water maze test was conducted as previously described with minor modification.33,34) Briefly, mice were trained to find a transparent Plexiglas platform in the pool placed 2 cm below the water surface in the middle of one quadrant. The position of the platform was unchanged during the training trials. Two time training trails per day were conducted for four consecutive days from 24 d after the first treatment of osthole. In each trial, the latency to escape on the platform was recorded for 120 s. Data of each mice behavior were collected by a video camera linked to a computer through an image analyzer. The average data of latency and searching distance for the platform in two trials of each mouse was counted for all tested mice per group per day. On the day 5, mice were tested on a navigation test, the latency and searching distance for the platform were recorded. On day 6, mice were tested on a spatial probe trial in which the platform was removed from the pool, and each mouse was allowed to swim freely for 120 s. During the probe trial, the time spent in the target quadrant and the number of platform crossings were recorded. The recorded data were used to analyze mice performance.
BrdU Labeling and ImmunohistochemistryIn order to assess the neurogenesis in mouse hippocampus, all mice were injected with 50 mg/kg of 5-bromo-deoxyuridine (BrdU; Sigma, Milan, Italy) (in PBS, pH 7.2) i.p. every 12 h for their last 7 d of survival. The mice were sacrificed 18 h after the last BrdU injection.35) The brain was harvested, and the sections were prepared.
For assessment of BrdU incorporation, sections were incubated for 30 min in 2 N HCl at 37°C to denature DNA, rinsed with PBS for three times, and blocked for 1 h at room temperature (RT) in 3% bovine serum albumin (BSA) solution containing 0.5% Triton X-100 (Sigma). The sections were then incubated with rat anti-BrdU antibody (1 : 400, Abcam, Cambridge, U.K.) and rabbit anti-NeuN antibody (1 : 500, EMD Millipore, Billerica, MA, U.S.A.) overnight at 4°C. The primary antibody was washed out with PBS three times. Sections were then incubated with Cy3 or fluorescein isothiocyanate (FITC)-conjugated species-specific secondary antibodies (all from Jackson ImmunoResearch Lab, West Grove, PA at 1 : 200 dilution) for 60 min at room temperature, followed by washing with PBS three times. Immunofluorescence controls were routinely performed with incubations in which primary antibodies were not included. Slides were covered with mounting medium (Vector Laboratories, Burlingame, CA, U.S.A.). Cell counter of ImageJ software (NIH ImageJ) was used to count BrdU-positive cells, and mean numbers were used for analysis. Results were visualized by fluorescent microscopy. Quantification of positive cells was performed on 5 sections per mouse, and 4 mice per group were analyzed.
For detection of neurogenesis in neonatal mouse brains of WT and APP/PS1 mice before deposition of Aβ peptide occurs in the brain, using the detection of proliferation marker Ki67 and neural stem cell marker Nestin. Briefly, the ice sections were prepared from neonatal brains of WT and APP/PS1 mice 1 d postnatal. Sections were fixed with 4% paraformaldehyde and rinsed with PBS for 3 times, then blocked for 1 h at RT in 3% BSA solution containing 0.5% Triton X-100 (Sigma). The sections were then incubated with rabbit anti Ki67 antibody (1 : 50, Abcam) and rat anti Nestin antibody (1 : 100, Chemicon, Temecula, CA, U.S.A.) overnight at 4°C. Sections were then incubated with Cy3 or FITC-conjugated species-specific secondary antibodies (all from Jackson ImmunoResearch Lab, West Grove, PA at 1 : 200 dilution) for 60 min at RT. The nuclei were stained by 4′-6-diamidino-2-phenylindole (DAPI). Slides were covered with mounting medium (Vector Laboratories, Burlingame, CA, U.S.A.). Fluorescence was recorded on a fluorescence microscope. ImageJ software (NIH ImageJ) was used to scan the pixel of the Nestin and Ki67 in 20× objective images. The ratio of Ki67/Nestin was calculated. All data was presented as relative of 100 control. n=4 mice were measured in each group, 5 sections from each animal were used for quantitative analysis.
For detection of Aβ(1–40) plaque formation in the hippocampus of APP/PS1 mice, brains were harvested after osthole treatment, and fixed in 4% paraformaldehyde overnight at 4°C, and paraffin sections (4 µm) were prepared for immunohistochemistry. Sections were dewaxed in xylene and rehydrated in a series of graded alcohols, then boiled in citrate buffer (10 mM, pH 6.0) for 20 min by microwave oven for antigen retrieval. Then the sections were treated with 3% H2O2 in PBS for 20 min at RT to abolish endogenous peroxidase activity. After blocking by goat serum, sections were incubated with rabbit anti Aβ(1–40) antibody (1 : 500, Thermo, Waltham, MA, U.S.A.). Sections were then incubated with biotinylated goat anti-rabbit immunoglobulin G (IgG) (1 : 200, Maixin, China) at 37°C for 30 min followed by streptavidin–peroxidase conjugate (1 : 200, Maixin, China). Aβ(1–40) plaques were visualized by staining sections with 3′-diaminobenzidine (DAB). The images were captured by microscope and Image J software (NIH ImageJ) was used to scan the pixel of the plaques. 5 sections from each animal were used for quantitative analysis (n=4 mice for each group).
Western BlotTotal proteins were extracted from hippocampal tissues of control (n=3) and osthole-exposed (n=3) mice by using ice cold RIPA buffer (Pierce, Rockford, IL, U.S.A.) containing 50 mM Tris (pH 8.0), 150 mM NaCl, 1 mM ethylenediamine tetraacetic acid (EDTA), 1% sodium deoxycholate, 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), and 1× protease, phosphatase-1, and phosphatase-2 inhibitor cocktails (Sigma, St. Louis, MO, U.S.A.). The lysate was centrifuged (20000×g, 10 min, 4°C). Protein concentration of each sample lysate was determined using the BCA kit (Thermo Scientific, Rockford, IL, U.S.A.).
The lysates (7.5–20 mg total protein) were separated on 10% SDS-polyacrylamide gel electrophoresis (PAGE) gels (except for BDNF where 12% gels were employed) and transferred to 0.45 mm polyvinylidene difluoride (PVDF) membrane (Pall, Pensacola, FL, U.S.A.) for probing with antibodies as noted. Membranes were blocked, washed and incubated overnight at 4°C with one of the following primary antibodies: mouse polyclonal anti-BDNF (1 : 500, EMD Millipore, Billerica, MA, U.S.A.), rabbit polyclonal anti-TrkB (1 : 500, Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), rabbit polyclonal anti-pTrkB (Tyr706) (1 : 400, Santa Cruz Biotechnology), Rabbit monoclonal anti-CREB (1 : 1000, Cell Signaling Technology, Boston, MA, U.S.A.), mouse polyclonal anti-phosphor-Ser133-CREB (1 : 1000, EMD Millipore, Billerica, MA, U.S.A.). After that, membranes were washed with 0.1% Tween 20, and then treated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, U.S.A.) for 1 h at 37°C. Peroxidase activity was visualized with an enhanced chemiluminescence substrate system (ECL, Santa Cruz Biotechnology) and exposure to film. Stripping filters and reprobing for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was carried out for normalization. Controls for nonspecific binding were determined by omission of the primary antibody. The intensity of the blots on films was quantified with densitometry.
Statistical AnalysisData were presented as the arithmetic mean±standard deviation (S.D.). Comparison between two groups was analyzed using Student t-test. Comparison between multi-groups was analyzed using the two-way ANOVA test. The data was analyzed by two-way ANOVA of genotype and treatment as the fixed factors. The Fg (F of genotype) value was used to detect the differences among WT and APP/PS1 groups. The Ft (F of treatment) value was used to detect the differences among groups with or without osthole treatment. The Fi (F of interaction) value was used to detect the interaction between effect of osthole and mouse genotype. A p values of less than 0.05 were considered statistically significant.
To investigate whether osthole treatment improved learning and memory in APP/PS1 mice, behavioral tests such as passive avoidance test (PAT) and Morris water maze (MWM) were performed following osthole treatment.
In PAT, the latency and the frequency of entering the dark compartment were recorded to evaluate the learning and memory performance of mice. Compared with WT group, the latency to enter the dark compartment in the APP/PS1 group was significantly decreased (Fg=16.996, p<0.01, Fig. 1A), and the frequency of entering the dark compartment was increased significantly (Fg=13.762, p<0.01, Fig. 1B), suggesting that the learning and memory performance of APP/PS1 transgenic mice were impaired. Compared with the APP/PS1 group, the latency was increased significantly (Ft=6.755, p<0.05; Fi=9.285, p<0.05, Fig. 1A), and the frequency of entering the dark compartment was decreased significantly (Ft=6.762, p<0.05; Fi=6.857, p<0.05, Fig. 1B) after osthole treatment, suggesting that osthole treatment ameliorated the learning and memory impairment in APP/PS1 mice.

Mice were divided into WT control mice, APP/PS1 transgenic mice, osthole-treated APP/PS1 mice and osthole-treated WT mice groups. In osthole-treated group, each animal was administered osthole via i.p. at a dosage of 30 mg/kg body weight once a day for 28 d. After 4 weeks of the osthole treatment, mice were subjected to a passive avoidance test. (A) The latency to enter the dark compartment was increased by osthole treatment as compared with APP/PS1 mice, and (B) the frequencies of entering the dark compartment was reduced by osthole treatment as compared with APP/PS1 mice. n=15 in each group. ## p<0.01, compared with WT group; * p<0.05, compared with APP/PS1 group.
Further, MWM test was performed. On the first day of the navigation test in MWM, we observed that the WT mice, APP/PS1 mice and osthole-treated APP/PS1 mice had a similar escape latency (p>0.05, Fig. 2A) and path length (p>0.05, Fig. 2B), indicating that the motility or vision of mice was not affected before the navigation test and probe trial. During the navigation test, The escape latency (Fig. 2A) and the path length (Fig. 2B) were reduced by osthole treatment as compared with APP/PS1 mice. On the day 5 of the navigation test (the testing progress), the APP/PS1 group showed higher escape latency (Fg=114.075, p<0.01, Fig. 2D), longer path length (mean distance to goal, Fg=527.975, p<0.01, Fig. 2E) than the WT group. While the escape latency and the path length in the osthole-treated APP/PS1 group were significantly reduced, compared to those in the APP/PS1 group (Ft=5.381, p<0.05; Fi=7.164, p<0.05, escape latency, Fig. 2D; Ft=15.919, p<0.01; Fi=21.511, p<0.01, mean distance to goal, Fig. 2E), suggesting that osthole treatment ameliorated the impairment of spatial acquisition of APP/PS1 mice. Then, the platform was removed in the probe trial on the 6th d of MWM tests, the APP/PS1 mice spent less time in the perimeter of the former position of the hidden platform in the target quadrant (Fg=58.789, p<0.01, Fig. 2F), and had fewer times passing through the original position of the hidden platform, compared with WT mice (Fg=82.970, p<0.01, Fig. 2G). In the osthole-treated APP/PS1 group, the time spent in target quadrant (Ft=7.980, p<0.05; Fi=7.420, p<0.05, Fig. 2F) and the frequencies to cross the former platform (Ft=28.015, p<0.01; Fi=16.500, p<0.01, Fig. 2G) significantly increased compared to the APP/PS1 mice. These results of MWM tests demonstrated that osthole treatment ameliorated the impairment in spatial learning function of APP/PS1 mice.

Mice were divided into WT control mice, APP/PS1 transgenic mice, osthole-treated APP/PS1 mice and osthole-treated WT mice groups. After 28 d administration of osthole or saline, mice were subjected to Morris water maze test. (A–B and D–E) The navigation test. In osthole-treated group, each animal was administered osthole via i.p. at a dosage of 30 mg/kg body weight once a day for 28 d. At day 24 post osthole first treatment, mice were training for searching the hiding platform in the Morris water maze for 5 d. The escape latency (A) and the path length (B) were reduced by osthole treatment as compared with APP/PS1 mice in the training process. Osthole treatment reduced the escape latency (D) and the path length (E) on the day 5 of training. (C) The representive locus plot after osthole treatment in navigation test and probe trial. (F–G) The probe test. (F) Osthole treatment prolonged the time spent in target quadrant. (G) Osthole treatment increased the frequencies of passing through the goal. n=15 in each group. ## p<0.01, compared with WT group; * p<0.05, ** p<0.01, compared with APP/PS1 group.
Next, we detected the Aβ(1–40) plaque formation in the APP/PS1 mice to ensure these mice suffering a similar pathological process of AD and to investigate whether osthole treatment affect the deposition of Aβ(1–40). After 28 d administration of osthole, the Aβ(1–40) plaques in the osthole-treated APP/PS1 mice did not change as compared with the APP/PS1 group (p>0.05, Figs. 3A, B), suggesting that osthole treatment did not decrease the deposition of Aβ(1–40) in the hippocampus in APP/PS1 mice. Thus, the therapeutic effect of osthole on cognitive dysfunction is associated with other possible mechanism.

Mice were divided into WT control mice, APP/PS1 transgenic mice and osthole-treated APP/PS1 mice groups. (A) After treatment of osthole for 28 d, the brains were removed and fixed in 4% paraformaldehyde overnight at 4°C, and routine paraffin sections (4 µm) were prepared for immunohistochemistry. Sections were dewaxed in a series of graded alcohols and boiled in citrate buffer, then were treated with 3% H2O2 to abolish endogenous peroxidase activity. After that immunohistochemical staining was performed by DAB labeled to detect the deposition of amyloid-β peptide (n=6 mice in each group). Scale bar: 50 µm. (B) Quantitative analysis showed that osthole treatment did not change the expression and deposition of Aβ(1–40) in the hippocampus of APP/PS1 mice. ## p<0.01, compared with WT mice.
Before the detection of effect of osthole on hippocampal neurogenesis in old APP/PS1 mice. We firstly performed immunohistochemisty to examine the neurogenesis in the brain before the formation of Aβ plaque in hippocampus, using the detection of proliferation marker Ki67 and neural stem cell marker Nestin. The Ki67 protein is strictly associated with and may be necessary for cellular proliferation. The protein is present during all active phases of the cell cycle (G1, S, G2, and mitosis), but is absent from resting cells (G0), and thus is widely used as an excellent marker to determine the growth fraction of a given cell population.31,36) As shown in Figs. 3A and B, the proliferation of neural stem cells (Ki67+/nestin+cells) in the neonatal brain did not differ between two groups, indicating that the cognitive dysfunction of APP/PS1 transgenic mice was associated with the formation of Aβ plaques.
To determine the effects of osthole on neurogenesis in hippocampus of APP/PS1 mice, we treated WT mice, APP/PS1 mice and osthole-treated APP/PS1 mice with BrdU i.p. for 7 d. The mice were sacrificed and the frozen sections of hippocampus were prepared for examination of neurogenesis. The labeled cells in the DG were visualized by immunostaining with antibody against BrdU and NeuN (Figs. 4C–E). The number of BrdU-positive cells in the DG of APP/PS1 mice (Fig. 4D) was markedly lower than that in the DG of WT mice (Fig. 4C), while the BrdU-positive cell number was increased in the DG of osthole-treated APP/PS1 mice (Figs. 4E) compared to APP/PS1 mice. Quantitative analysis was performed to determine the number of BrdU-positive cells in DG of hippocampus. As shown in Fig. 4F, APP/PS1 mice exhibited lower BrdU+ in the sections than that in WT group (20.33±2.08 vs. 53.45±7.09, Fg=64.764, p<0.01). Whereas, the number of BrdU+ cells in the sections of osthole-treated APP/PS1 mice was higher than that in APP/PS1 mice (31.26±4.58 vs. 20.33±2.08, Ft=6.557, p<0.05), indicating that osthole treatment can enhanced neurogenesis in hippocampus of APP/PS1 mice.

(A) Neurogenesis in neonatal brains of WT mice and APP/PS1 mice. Brains were harvested 1 d postnatal from WT and APP/PS1 mice, immunocytochemistry was performed to detect the expression of Nestin (a marker of neural stem cells) and Ki67 (a marker for cellular proliferation). The nuclei were stained by DAPI. (B) Quantitative analysis showed that expression of Nestin and the number of Ki67 positive neural stem cells did not differ between WT and APP/PS1 neonatal mice. (C–E) Representative BrdU/NeuN double-labeled images in the DG of the hippocampus in mice. Mice were divided into WT control mice, APP/PS1 transgenic mice and osthole-treated APP/PS1 mice groups. BrdU (50 mg/kg) was injected into all mice for 7 d before sacrificed. After 28 d treatment of osthole, brain sections (hippocampus) were prepared. Sections were fixed by 4% paraformaldehyde and blocked by 3% BSA solution containing 0.5% Triton X-100. Immunostaining with antibody against BrdU to identify proliferating cells in the hippocampus of WT mice, APP/PS1 mice or of osthole treated APP/PS1 mice. Nuclei of neurons are stained with NeuN to show the DG structure. Fluorescence was recorded on a fluorescence microscope. There were less BrdU-positive cells (white arrows) in DG of the APP/PS1 mice (D) by compared with WT mice (C), whereas increased BrdU+/NeuN cells were found in the hippocampus of APP/PS1 mice treated with osthole (E) by compared with APP/PS1 mice. (F) Quantification shows the distribution of proliferating cells in the dentate gyrus of hippocampus. ImageJ software (NIH ImageJ) was used to scan the pixel of the Nestin and Ki67 in 20× objective images. The ratio of Ki67/Nestin was calculated. Six sections for each mouse, 6 mice per group were evaluated. All data was presented as relative of 100 control. APP/PS1 mice exhibited lower BrdU+ in the sections than that in WT group, whereas, the number of BrdU+ cells in the sections of osthole-treated APP/PS1 mice was higher than that in APP/PS1 mice. ##p<0.01, compared with WT group; * p<0.05, compared with APP/PS1 group.
BDNF, a most common neurotrophic factor, contributes to the enhancement of adult hippocampal neurogenesis.19) Therefore, we investigated the influence of osthole on BDNF levels in the hippocampus by Western blot. As shown in Figs. 5A and B, osthole significantly increased BDNF levels (Ft=11.902, p<0.05) in APP/PS1 mice, indicating that the inductive effect of osthole on the neurotrophic factor, which may be beneficial for neurogenesis.
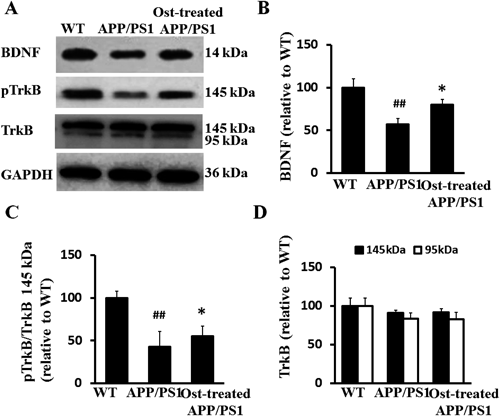
Mice were divided into WT control mice, APP/PS1 transgenic mice and osthole-treated APP/PS1 mice groups. After the behavioral tests, the hippocampus was harvested from each group and Western blot was performed to analyze the levels of BDNF, TrkB and pTrkB. (A) Representative Western blot images of BDNF, pTrkB and TrkB expression in different groups. (B) Quantitative analysis of BDNF. By compared with WT mice, BDNF expression was significant lower in APP/PS1 mice. Osthole treatment increased the BDNF level in hippocampus of APP/PS1 mice. (C) Quantitative analysis of pTrkB/TrkB 145 kDa. The pTrkB/TrkB ratio was decreased in APP/PS1 mice compared to WT mice, while osthole treatment significantly increased the ratio compared to APP/PS1 mice. (D) Quantitative analysis of 145 kDa and 95 kDa TrkB. The differences of the expression of 145 kDa TrkB and 95 kDa TrkB were not statistically significant in hippocampus among goups. GPADH was used as a loading control. The integrated density value of the bands in western blots was determined using densitometry, and data was normalized to GAPDH and to control (WT mice). n=3 in each group. ## p<0.01, compared with WT group; * p<0.05, compared with APP/PS1 group. Note that osthole significantly increased the expression of BDNF but not of 145 kDa TrkB and 95 kDa TrkB, while osthole activated 145 kDa TrkB to phosphorylation.
As TrkB is the high-affinity receptor of BDNF in hippocampus, we then examined the expression of both full-length (145 kDa) and truncated (95 kDa) TrkB receptors, depicted in Fig. 5A. The differences of the expression of 145 kDa TrkB and 95 kDa TrkB were not statistically significant in hippocampus of WT mice, APP/PS1 mice and osthole-treated APP/PS1 mice (p>0.05, Fig. 5D), suggesting that in APP/PS1 mice, total TrkB levels are not altered compared to WT mice, and osthole does not affect TrkB expression in hippocampus of APP/PS1 mice.
Numerous studies in FAD mouse models suggest that misregulated TrkB activity plays a critical role in the pathogenesis of AD.37,38) One possibility is that TrkB activity is compromised while its level of expression is unaltered. To evaluate this, we used an antibody that recognizes phosphorylated TrkB, which corresponds to the inactive form of TrkB 145 kDa. To determine whether osthole acts on compromised TrkB signaling pathway in APP/PS1 mice, we compared the ratio of pTrkB 145 kDa to total TrkB 145 kDa expression levels in WT mice, APP/PS1 and osthole-treated APP/PS1 mice by Western blot analysis. The pTrkB/TrkB ratio was decreased in APP/PS1 mice compared to WT mice (Fg=119.492, p<0.01, Fig. 5C), while osthole treatment significantly increased the ratio compared to APP/PS1 mice (Ft=6.574, p<0.05, Fig. 5C), indicating that the inductive effect of osthole on the activation of TrkB.
Osthole Treatment Increased CREB Expression and Enhanced CREB PhosphorylationIn order to explore the role of CREB and p-CREB in osthole mediated regulation of BDNF-TrkB signaling, we further determined their expression levels in hippocampus of WT mice, APP/PS1 mice and osthole-treated APP/PS1 mice (Figs. 6A, B). Quantitative analysis was performed to determine the intensity of CREB/GAPDH and pCREB/GAPDH. The levels of CREB in APP/PS1 mice showed a decrease (Fg=37.851, p<0.01, Fig. 6C) compared to WT mice, and osthole treatment slightly increased the CREB expression (not significant) compared to APP/PS1 mice. However, osthole treatment significantly increased the phosphorylated CREB levels in APP/PS1 mice (Ft=27.120, p<0.05, Fig. 6C), indicating that osthole treatment activates BDNF-TrkB signaling by activation of CREB.

After the behavioral tests, the hippocampus was also harvested to do Western blot analysis for the detection of CREB and pCREB expression. (A) Representative Western blot images of CREB and (B) pCREB expression in different groups. (C) Quantitative analysis of CREB and pCREB. GPADH was used as a loading control. The integrated density value of the bands in Western blots was determined using densitometry, and data was normalized to GAPDH and to control (WT mice). The levels of CREB in APP/PS1 mice showed a decrease compared to WT mice, and osthole treatment slightly increased the CREB expression (not significant) compared to APP/PS1 mice. Whereas, osthole treatment significantly increased the phosphorylated CREB levels in APP/PS1 mice. n=3 in each group. ## p<0.01, compared with WT group; * p<0.05, compared with APP/PS1 group.
Our study provided collective evidence indicating that osthole treatment increased adult hippocampal neurogenesis in APP/PS1 mice by stimulating the secretion of BDNF, activation of TrkB and CREB phosphorylation in hippocampus, resulting in an amelioration of impairments on spatial learning and memory.
The APP/PS1 transgenic mouse model is a widely used model of AD, co-expressing Swedish, mutated human APP695 and human mutated PS1 in which exon 9 is deleted. It mimics the pathological and behavioral changes of AD based on amyloid hypothesis. Amyloid peptide (Aβ) is derived from the cleavage of amyloid precursor protein (APP). APP undergoes ectodomain shedding at two alternative sites, one is mediated by BACE1 and results in the formation of membrane-associated C-terminal fragments (CTFs), termed C99s. The subsequent PS/γ-secretase-mediated cleavage of C99 at the γ-site releases Aβ peptides of different lengths.39,40) The abnormal accumulation of Aβ forms extracellular senile plaques in AD brain and is considered as the main cause of inflammation and oxidative stress in the brain, resulting in neural loss and cognitive dysfunction.13,41,42) Additionally, cognitive dysfunction and severe impairment of neurogenesis in the SGZ of the DG has been reported in APP/PS1 mice.13,14) Memory impairments were seen as early as 7 months of age,43) thus, in the present study, we used APP/PS1 mice at age of 8–9 months. Osthole, an active constituent of Angelica Pubescentis Radix (R. angelicae pubescentis) and Cnidium monnieri (L.) CUSSON, has been reported to exert neuroprotective effects21–26) and possess the capable of improvement of neural stem cell proliferation.27) Recently, osthole also has been reported to reverse Aβ neurotoxicity by stimulating BDNF production and enhancing CREB phosphorylation in cultured cortical neurons.21) However, the therapeutic effects of osthole on AD animals are largely unknown. Our results showed that osthole treatment improved the learning and memory impairment in APP/PS1mice in MWM tests and PAT, decreasing the escape latency and the path length to goal, prolonging the time spent in target quadrant and increasing the times of passing through the goal, indicating that osthole treatment ameliorated the cognitive deficits in APP/PS1 transgenic mice. However, further behavior test should be performed to confirm the therapeutic effect of osthole on cognitive dysfunction in other model such as an intrinsic aging mouse model.
The hallmarks of AD pathologies are extracellular deposition of Aβ protein as senile plaques and neurofibrillary tangles (NFTs) intra neurons formed as a result of abnormal hyperphosphorylation of cytoskeletal tau protein. These pathologies of AD are evident in specific, vulnerable brain areas and the hippocampus is one of the earliest to be affected.44) The hippocampus is a mammalian brain structure that lies under the medial temporal lobe, with one on each side of the brain. The hippocampus is one of the neurogenesis area in the mammalian CNS. Besides the SVZ, neurogenesis occurs in the SGZ of DG in the hippocampus. A growing body of evidence supports the view that promotion of adult hippocampal neurogenesis improves pattern separation and spatial memory.12,45) In contrast, a decline in neurogenesis may underlie cognitive impairments associated with aging and disorders such as AD.9,46) In the present study, we firstly found that neurogenesis in neonatal brains of APP/PS1 mice did not differ from WT mice, indicating that the formation of Aβ plaque is associated with the cognitive dysfunction of APP/PS1 mice. Next, we detected the hippocampal neurogenesis in the WT mice, APP/PS1 mice and osthole-treated APP/PS1 mice by BrdU incorporation. Our results showed that the BrdU-positive cells in the SGZ of DG in hippocampus significantly reduced compared to WT groups, while osthole treatment increased the number of BrdU+ cells in the DG of hippocampus in APP/PS1 mice, suggesting that osthole conferred profound improvement of adult neurogenesis of hippocampus, which might be one of the underlying mechanisms for the therapeutic potential on AD.
There is increasing interest in the relationship between BDNF/TrkB signaling and adult hippocampal neurogenesis.18) It has been found that endogenous upregulation of BDNF contributes to the behavioral effect of antidepressants through enhancement of adult hippocampal neurogenesis,19) and the secretion of BDNF by various types of stem cells was proposed as an important mechanism for their ability to counteract cognitive and neuropathological symptoms of AD in transgenic models.47) Therefore, the therapeutic strategy to stimulate BDNF production has potential for modification of neurodegenerative diseases such as AD. The results of the present study demonstrate that osthole treatment induced a significant increase of BDNF levels in the APP/PS1 mice, indicating that osthole augments neurogenesis by enhancing BDNF production in hippocampus. There are two TrkB receptor isoforms abundantly expressed in the brain: full-length and truncated TrkB.48) BDNF activates intracellular signaling cascades through full-length TrkB (145 kDa) to induce neural proliferation and survival.49) In the present study, we performed Western blot analysis to determine TrkB (145 kDa), TrkB (95 kDa) and pTrkB (145 kDa) levels in the hippocampus, we found that osthole treatment significantly increased the pTrkB/TrkB ratio in the APP/PS1 mice, indicating that osthole augments neurogenesis by enhancing TrkB phosphorylation. All these results suggested that osthole promoted hippocampal neurogenesis in AD via BDNF activating TrkB signaling.
CREB is a nuclear transcription factor that is activated by phosphorylation at serine133.50) Activation of CREB is essential for the formation and retention of memory, which is the main physiological parameter of Alzheimer’s disease.51) The process of CREB activation is also considered to be a major mechanism in the promotion of neural growth and survival.52) The activation of BDNF/TrkB signaling lead to the phosphorylation of CREB,20) which has been characterized to be a critical process for the transcriptional regulation of hippocampal neurogenesis. Loss of CREB function has been shown to dendrite growth and arborization of newborn hippocampal neurons.53) A number of studies reported that phosphorylated CREB (pCREB) is present in the vast majority of newly generated immature neurons of the SGZ of DG in hippocampus.54,55) Hence, we detected CREB and pCREB levels following the treatment of osthole in hippocampus of APP/PS1 mice. Our results indicated that the level of CREB in APP/PS1 group was lower than WT mice, but osthole did not increased the basal CREB expression significantly. Our results in this paper indicated that CREB expression decreased in APP/PS1 mice, which was consistent with the previous report. The mRNA level and protein expression of CREB were downregulated in Tg2576 mouse hippocampus, and the protein expression of CREB was also decreased in AD-postmortem brain.56) Further, we found that osthole treatment increased CREB phosphorylation in the hippocampus of APP/PS1 mice, indicating that osthole improves hippocampal neurogenesis by activating CREB through BDNF/TrkB signaling. However, there are several up streams of CREB/BDNF may be involved in neurogenesis, including PKA, TrkB-MAPK-RSK and CaM-CaMKIV.57) Further study should perform to detect other possible mechanism of CREB/BDNF pathway and other possible pathway such as Akt signaling since it is associated with survival and proliferation of neural stem cells.58)
In conclusion, osthole treatment ameliorated the impairment of learning & memory via augmenting hippocampal neurogenesis. The effects of osthole on phosphorylation CREB through activating the BDNF/TrkB signaling might be the possible underlying mechanisms of the enhancement of neurogenesis. These results suggested that osthole is a potential therapeutic agent to provide beneficial effects on aging and AD.
We thank Katherine Page for editorial assistance.
The authors declare no conflict of interest.