2015 Volume 38 Issue 10 Pages 1470-1477
2015 Volume 38 Issue 10 Pages 1470-1477
In our previous study, panaxytriol (PXT) was shown to enhance midazolam (MDZ) 1′-hydroxylation significantly but to inhibit MDZ 4-hydroxylation. To explore the underlying mechanism, we investigated the effects of PXT on cytochrome P450 3A (CYP3A)-mediated MDZ metabolic pathways using rat liver microsomes (RLM), human liver microsomes (HLM), and rat primary hepatocytes. In the presence of PXT, the Vmax of 4-OH MDZ decreased from 0.72 to 0.51 nmol/min·mg pro in RLM and from 0.32 to 0.12 nmol/min·mg pro in HLM, and the Km value increased from 5.12 to 7.26 µM in RLM and from 27.87 to 32.80 µM in HLM. But the presence of PXT reduced the Km and increased the Vmax values of MDZ 1′-hydroxylation in RLM and HLM. Interestingly, the differential effect of PXT on MDZ 4-hydroxylation and 1′-hydroxylation was also observed in primary rat hepatocytes after 45-min culture. PXT did not affect the expression levels of CYP3A1/2 mRNA in rat hepatocytes. With extension of the culture time to 6 h, however, PXT significantly inhibited both MDZ 4-hydroxylation and 1′-hydroxylation, and the expression level of CYP3A1/2 mRNA was decreased to 87% and 80% (CYP3A1) and to 89% and 85% (CYP3A2) of those in controls in the presence of PXT 4.0 and 8.0 µg/mL, respectively. These results suggest that PXT could activate MDZ 1′-hydroxylation but inhibit MDZ 4-hydroxylation by changing the CYP3A enzyme affinity and metabolic rate after a short-term intervention. However, long-term treatment with PXT could inhibit both the 4-hydroxylation and 1′-hydroxylation of MDZ by downregulating CYP3A1/2 mRNA expression.
Cytochrome P450 (CYP450) is a superfamily of hemeproteins that involved in the biotransformation of the majority of clinical drugs. The inhibition or induction effect of clinical drugs on CYP450 is considered as one of the important factors leading to potential drug–drug interactions.1–3) CYP3A4 is one of the primary CYP450 enzymes in human liver and catalyzes the metabolism of more than 50% of clinical drugs including terfenadine, theophylline and astemizole, etc. Midazolam (MDZ) is the specific substrate of CYP3A4 enzyme and is mainly metabolized to 4-hydroxymidazolam (4-OH MDZ) and 1′-hydroxymidazolam (1′-OH MDZ) by combining with the two different sites of CYP3A4 enzyme active center.4,5) Therefore, the formation of the two metabolites are often simultaneously quantified for the assessment of CYP3A4 activity.
Traditional Chinese medicine is widely used in clinical practice in China because of the advantages of good curative effect and less adverse reaction. The incidence of Chinese medicine and chemical medicine interactions significantly increased as the widely using of Chinese medicine, and the change of CYP450 enzyme activity is the principal cause resulting in drug metabolic interactions.6,7) As a well known study, salvia extract exhibited a marked inhibition effect on the expression of CYP3A4 and CYP1A2 mRNA in HepG2, and high concentration of salvia extract could restrain the induction of CYP3A4 mRNA mediated by rifampin.8) Astragalus membranaceus injection and granules can increase the expression of CYP3A1 mRNA in rats, and promote the metabolism of CYP3A1 probe drugs.9)
Panaxytriol (PXT) is one of the active ingredients in red ginseng extract and can inhibit the activity of some kind of cancer cells including human gastric carcinoma cells and human breast cancer cells.10) PXT is also an important active component in Shenmai injection which is extracted from red ginseng and ophiopogonis, and is widely used for the treatment of cardiovascular system diseases including coronary atherosclerotic cardiopathy and viral myocarditis, and is often co-administered with other clinical medicines. In our previous study, panaxytriol showed differential effect on the metabolic pathways of MDZ, significantly inscreasing MDZ 1′-hydroxylation but inhibiting MDZ 4-hydroxylation.11,12) This results revealed that PXT had the potential to inhibit or activate the CYP3A4 enzyme activity. Therefore, it is necessary to further study the impact mechanism of PXT on CYP3A4 enzyme and possible interaction with other clinical drugs.
The purpose of this study was to investigate the effect mechanism of PXT on CYP3A4-mediated MDZ hydroxylation metabolism, CYP3A4 enzyme activity and expression by rat liver microsomes (RLM), human liver microsomes (HLM) and primary rat hepatocytes.
Rats (180–200 g) were obtained from the Animal Experimental Center of Nanchang University and the animal study protocol was approved by the Review Committee of Animal Care. PXT was provided by Xian sinuote biological technology Co., Ltd. (Xian, China). Dexamethasone (DEX) and ketoconazole (KTZ) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). MDZ, 1′-OH MDZ, 4-OH MDZ, NADP+, glucose 6-phosphate, glucose-6-phosphate dehydrogenase, 3-(4,5-dimetylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). HLM and RLM were purchased from the Research Institute for Liver Diseases Co., Ltd. (Shanghai, China). EasyScript™ First-Strand cDNA Synthesis SuperMix was provided by Beijing TransGen Biotech Co., Ltd. (Beijing, China). HPLC-grade acetonitrile was obtained from Merck Co. (Darmstadt, Germany).
Effect of PXT on MDZ Metabolic Kinetics Mediated by CYP3A in MicrosomesTo investigate the effect of PXT on metabolic kinetics of MDZ mediated by CYP3A in RLM (0.2 mg/mL) and HLM (0.2 mg/mL), the production of 1′-OH-MDZ and 4-OH-MDZ was simultaneously determined in the presence of different concentrations of PXT (0, 0.5, 1, 2 µg/mL). The incubation mixtures (final volume: 200 µL) including liver microsomes and various concentrations of MDZ (1, 2, 5, 10, 20, 40, 80 µM) were preincubated for 5 min at 37°C. The reactions were initiated by adding an reduced nicotinamide adenine dinucleotide phosphate (NADPH)-regeneration system (0.5 mM NADP+, 10 mM glucose-6-phosphate, 1 unit/mL glucose-6-phosphate dehydrogenase and 5 mM MgCl2), and terminated by adding 200 µL of ice-cold acetonitrile at 10 min in RLM and 5 min in HLM. The samples were centrifugated at 20000×g for 10 min, and 20 µL of the supernatant was injected for HPLC-UV analysis. All experiments were conducted in triplicate.
Rat Hepatocytes PreparationHepatocytes were freshly isolated from rats by a two-step collagenase perfusion method according to Seglen with modifications.13) After isolation, the hepatocytes were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, 100 units/mL penicillin, 100 µg/mL streptomycin, and maintained at 37°C under a humidified atmosphere with 95 : 5% air/CO2. The yield and viability of hepatocytes were determined by 0.4% trypan blue exclusion test, and cells with the viability greater than 85% were used in this study.
Cell Toxicity AssessmentHepatocytes with high viability were seeded in collagen gel-precoated 96-well plate with a density of 1×105 cells/mL, then different concentrations of MDZ, KTZ, DEX and PXT were added to the medium, respectively. After incubating for 3 h, MTT solution (5 mg/mL) was added to each well and incubated with cells for 4 h. Then dimethyl sulfoxide (DMSO) was added to every wells after abandoning medium and the absorbance was measured at 490 nm with Enzyme-linked immune detector. Wells only containing the culture medium without the test drugs were used as blank control. The test drugs were deemed to have toxicity to cells when the absorbance significantly decreased.
Effect of PXT on MDZ Metabolism Mediated by CYP3A in Rat HepatocytesHepatocytes with viability greater than 85% were seeded in collagen gel-precoated 12-well plate at a density of 1×106/mL (2 mL each well) and cultured under 37°C with 95% humidity and 5% CO2. The culture medium contained MDZ (CYP3A substrate, 10 µM) and various concentrations of DEX (CYP3A inducer, 5, 10, 20 µM), or KTZ (CYP3A inhibitor, 2, 4, 8 µM), or PXT (2, 4, 8 µg/mL). DEX and KTZ were used as the positive control and negative control, respectively. All the stock solutions of the tested compounds were dissolved in DMSO, and the final concentration of the solvent in each well was lower than 0.1% (v/v). After 45 min, 4 h and 6 h incubation, the reaction was terminated by collecting 150 µL of the culture medium to −80°C. After unfreezing and centrifugating, 100 µL of the supernatant was separated and then 200 µL acetonitrile containing internal standard gliclazide (450 ng/mL, 20 µL) was added for protein precipitation. The samples were then briefly vortexed and centrifugated at 20000×g for 10 min, and 5 µL of the supernatant was injected for LC-MS analysis.
Isolation of RNA and Reverse Transcription-Polymerase Chain Reaction (RT-PCR) AnalysisHepatocytes were seeded in 60 mm petri dish at a density of 4×105/mL under 37°C with 95% humidity and 5% CO2. After culturing for 45 min or 6h in the presence of DEX (20 µM) or different concentrations of PXT (2, 4, 8 µg/mL), the culture media was abandoned and the cells were dissolved in 1-mL of TRIzol reagent. The total RNA was isolated according to the manufacturer’s protocols. The integrity of RNA was detected by 1% agarose gel electrophoresis. RNAs were reverse-transcribed into cDNA by using EasyScript™ RT reagent Kit, PCR and 2% agarose gel electrophoresis was performed to separate and relatively quantify the target gene. Table 1 showed PCR primers for rat CYP3A1, CYP3A2 and β-actin gene.
| Gene | Forward primer (5′→3′) | Reverse primer (3′→5′) | Fragment size |
|---|---|---|---|
| β-Actin | TCAGGTCATCACTATCGGCAAT | AAAGAAAGGGTGTAAAACGCA | 432 |
| CYP3A1 | ATCCGTTGTTCTTGTCAGTAG | AGTATCATAGGTGGGAGGTG | 401 |
| CYP3A2 | TGGACCCAGGAACTGCATTG | CCATGCATCAAGAGCAGTCAA | 329 |
The concentrations of 1′-OH-MDZ and 4-OH-MDZ in microsomes were determined using an HPLC-UV method. Separation of the metabolites of MDZ was performed on a diamond C18 column (150 mm×4.6 mm, i.d. 5 µM) with the mobile phase of 20 mM ammonium acetate and acetonitrile (62 : 38, v/v) at a flow rate of 1.0 mL/min. The metabolites were detected at 254 nm wavelength using an ultraviolet detector. No obvious interferences from the incubation system were observed under these conditions. The retention time of 4-OH-MDZ and 1′-OH-MDZ were 6.2 and 8.0 min, respectively.
The metabolites of MDZ in hepatocytes were determined using an LC-MS method. LC-MS analysis was performed on a Shimadzu Pack VP-ODS C18 (150 mm×2.0 mm, i.d. 5 µM) with the mobile phase of 0.01% ammonium acetate (A) and acetonitrile (B). A gradient elution was used as flows: 0.03–5.0 min, 30–90% (B); 5.0–6.0 min, 90–30% (B); 6.0–11.0 min, 30–30% (B), and the flow rate was set at 0.2 mL/min. Quantification of 4-OH MDZ (m/z 342.00), 1′-OH MDZ (m/z 342.00) and gliclazide (m/z 324.15, internal standard) was performed in selected positive ion monitoring mode, and the retention time were 6.3, 6.7, and 8.1 min, respectively.
Data AnalysisAll values were presented as mean±standard deviation (S.D.) of three independent experiments. The enzymatic kinetic parameters were calculated by Michaelis–Menten equation. Unpaired t-test was used to compare CYP3A activity and mRNA levels between different groups. Data were considered to be statistically significant when p<0.05.
The effects of different concentrations of PXT on MDZ metabolism in RLM and HLM were showed in Figs. 1 and 2, respectively. In RLM and HLM, PXT differentially affected CYP3A-mediated 4-hydroxylation and 1′-hydroxylation of MDZ, inhibiting 4-hydroxylation metabolism but activating 1′-hydroxylation metabolism. The inhibition and activation effect of PXT on MDZ metabolism presented a concentration-dependent characteristic. The kinetics parameters were presented in Table 2 (4-OH MDZ) and Table 3 (1′-OH MDZ). The kinetics parameters (maximum reaction velocity: Vmax and Michaelis constant: Km) of MDZ 4-hydroxylation and 1′-hydroxylation were calculated by Michaelis–Menten equation (Eq. 1) and a two-site model equation (Eq. 2), respectively. Vmax of MDZ 4-hydroxylation was decreased from 0.72 to 0.51 (nmol/min·mg protein), Km was increased from 5.12 to 7.26 µM and Clint (Vmax/Km) was decreased from 0.14 to 0.07 (mL/min·mg pro) with the increase of PXT concentration in RLM. Vmax of MDZ 1′-hydroxylation was increased from 0.38 to 0.84 (nmol/min·mg protein), Km1 was decreased from 8.92 to 5.46 µM and Clint (Vmax1/Km1) was increased from 0.04 to 0.16 (mL/min·mg pro) with the increase of PXT concentration in RLM. The kinetics parameters (Vmax, Km and Clint) of the 4-hydroxylation and 1′-hydroxylation of MDZ presented the similar trend in HLM. These results indicated that PXT inhibited MDZ 4-hydroxylation but activated its 1′-hydroxylation in RLM and HLM.
 | (1) |
 | (2) |

The concentration of MDZ used for enzyme kinetics were 1, 2, 5, 10, 20, 40, and 80 µM, and the concentration of PXT were 0, 0.5, 1.0, and 2.0 µg/mL.

The concentration of MDZ used for enzyme kinetics were 1, 2, 5, 10, 20, 40, and 80 µM, and the concentration of PXT were 0, 0.5, 1.0, and 2.0 µg/mL.
| PXT (µg/mL) | Vmax (nmol/min·mg pro) | Km (µM) | Vmax/Km (mL/min·mg pro) | |
|---|---|---|---|---|
| RLM | 0 | 0.72±0.09 | 5.12±0.61 | 0.14±0.02 |
| 0.5 | 0.63±0.08 | 5.25±0.54 | 0.12±0.02 | |
| 1.0 | 0.57±0.08 | 6.47±0.73 | 0.09±0.01 | |
| 2.0 | 0.51±0.05 | 7.26±0.71 | 0.07±0.01 | |
| HLM | 0 | 0.32±0.04 | 27.87±3.52 | 11.48±1.31 |
| 0.5 | 0.24±0.04 | 28.23±3.73 | 8.50±1.11 | |
| 1.0 | 0.17±0.03 | 30.18±3.42 | 5.63±0.93 | |
| 2.0 | 0.12±0.02 | 32.80±3.14 | 3.66±0.42 |
| PXT (µg/mL) | Vmax1 (nmol/min·mg pro) | Km1 (µM) | Vmax1/Km1 (mL/min·mg pro) | Vmax2/Km2 (µL/min·mg pro) | |
|---|---|---|---|---|---|
| RLM | 0 | 0.38±0.08 | 8.92±0.62 | 0.04±0.01 | 7.13±1.13 |
| 0.5 | 0.49±0.07 | 8.61±0.92 | 0.06±0.01 | 6.21±0.81 | |
| 1.0 | 0.58±0.09 | 5.82±0.63 | 0.10±0.02 | 7.83±0.74 | |
| 2.0 | 0.84±0.10 | 5.46±0.71 | 0.16±0.02 | 6.42±0.81 | |
| HLM | 0 | 0.53±0.08 | 5.91±0.71 | 0.09±0.01 | 7.62±0.91 |
| 0.5 | 0.98±0.11 | 5.83±0.63 | 0.17±0.02 | 4.22±0.63 | |
| 1.0 | 1.12±0.16 | 5.53±0.81 | 0.20±0.04 | 5.31±0.54 | |
| 2.0 | 1.35±0.15 | 5.21±0.62 | 0.26±0.03 | 7.92±0.83 |
MDZ is a probe substrate of CYP3A. The chang of CYP3A activity was revealed by the concentrations of 4-OH MDZ and 1′-OH MDZ in the culture medium. The results of cell toxicity assessment showed that MDZ in the concentration range of 1–40 µM and PXT in the concentration range of 0.5–20 µg/mL had no toxicity to rat hepatocytes. The effect of PXT on MDZ metabolism in rat hepatocytes was showed in Fig. 3 (4-OH MDZ) and Fig. 4 (1′-OH MDZ). After culturing for 45 min with PXT (2, 4, 8 µg/mL), the production of 4-OH MDZ was decreased to 93.7, 82.5, 70.1% of that in control, while the generation of 1′-OH MDZ was increased to 106.0, 132.9, 138.1% of that in control. The result showed PXT had differential effect on the metabolic pathways of MDZ in rat hepatocytes, similar to the results obtained from RLM and HLM incubation system. After culturing for 6 h, however, PXT exhibited a significant inhibition effect, similar to KTZ, on both MDZ 4-hydroxylation and 1′-hydroxylation of MDZ in rat hepatocytes. The formation of 4-OH MDZ and 1′-OH MDZ in the presence of PXT (2, 4, 8 µg/mL) were 92.5, 54.1, 15.5 and 82.3, 68.2, 59.0% of that in control, respectively. These results showed PXT could enhance the inhibition on 4-hydroxylation of MDZ but reverse its activation on 1′-hydroxylation mediated by CYP3A with the extension of culture time in hepatocytes.

The concentrations were as follows: low (5 µM DEX, 1 µg/mL PXT, and 2 µM KTZ); middle (10 µM DEX, 4 µg/mL PXT, and 4 µM KTZ); high (20 µM DEX, 8 µg/mL PXT, and 8 µM KTZ); (n=3, mean±S.D., * p<0.05, ** p<0.01).
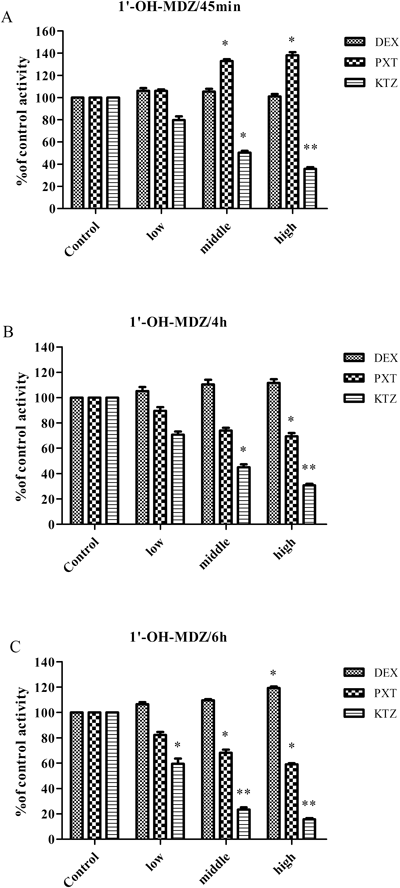
The concentrations were as follows: low (5 µM DEX, 1 µg/mL PXT, and 2 µM KTZ); middle (10 µM DEX, 4 µg/mL PXT, and 4 µM KTZ); high (20 µM DEX, 8 µg/mL PXT, and 8 µM KTZ); (n=3, mean±S.D., * p<0.05, ** p<0.01).
Base mRNA levels of CYP3A1/2 were analyzed by reverse transcriptase-polymerase chain reaction. All values were compared with those in blank group set as 100%. The main results were presented in Figs. 5 and 6. Different changes were observed in CYP3A1/2 mRNA expression according to culturing time in primary rat hepatocytes.

A: Control; B: 20 µM DEX; C: 2 µg/mL PXT; D: 4 µg/mL PXT; E: 8 µg/mL PXT.

A: Control; B: 20 µM DEX; C: 2 µg/mL PXT; D: 4 µg/mL PXT; E: 8 µg/mL PXT.
As expected, no obvious change of CYP3A1/2 mRNA expression were evidenced after culturing for 45 min in the presence of DEX and PXT. But after culturing for 6h in the presence of PXT (4.0, 8.0 µg/mL), the expression of CYP3A1 mRNA decreased to 87, 80% of that in control and CYP3A2 mRNA decreased to 89, 85% of that in control, respectively. Only a slight induction effect was observed by the addition of DEX after culturing for 6 h.
MDZ is the specific substrate of CYP3A4 enzyme, and primarily metabolised to 4-hydroxylation and 1′-hydroxylation metabolites mediated by CYP3A4. The present study revealed that different concentrations of PXT could inhibit 4-hydroxylation but activate 1′-hydroxylation of MDZ metabolism in RLM and HLM. In the presence of PXT in RLM, Vmax of 4-OH MDZ was decreased from 0.72 to 0.51 nmol/min·mg pro, and Km was increased from 5.12 to 7.26 µM. Likewise in HLM, Vmax of 4-OH MDZ was decreased from 0.32 to 0.12 nmol/min·mg pro, and Km was increased from 27.87 to 32.80 µM as the addition of PXT. These results indicated that PXT could inhibit 4-hydroxylation of MDZ by reducing the enzyme transform velocity and affinity.
On the contrary, the presence of PXT reduced Km and increased Vmax of MDZ 1′-hydroxylation in RLM and HLM. These results demonstrated that PXT could activate MDZ 1′-hydroxylation mediated by CYP3A through enhancing the enzyme affinity and metabolic rate.
Many studies proved that some CYP3A-mediated reactions exhibited atypical Michaelis–Menten kinetics including substrate activation, substrate inhibition and different kinetics.14–17) Apparently, the differential effect of PXT on MDZ metabolism is only able to be explaind using an atypical kinetics model. A hypothesis declared that CYP3A enzyme contains two or more MDZ-binding sites.18,19) The two-site model considered that PXT could bind to one defined site (site A) required for its 4-hydroxylation (or 1′-hydroxylation), and the binding of PXT to A site caused an allosteric effect on the other MDZ-binding site (site B) required for its 1′-hydroxylation (or 4-hydroxylation). However, the multi-site model hypothesized that, an activator binding site which is closely related to the substrate binding sites also exists in addition to substrate binding sites. PXT can bind to the activator binding site, leading to conformational transitions in CYP3A4, and ultimately to differential effects on MDZ metabolism. The induction and inhibition effect on CYP450 of medicines was the major mechanism resulting in drug–drug interaction, further work was necessary to study the influence on CYP3A enzyme of PXT.
Traditionally, the liver plays a major role in the metabolism and transformation of many compounds. Our preliminary experiments indicated that hepatic cell lines including HepaRG and human embryo L-02 cells were under restrictions as the weak metabolic function. Primary rat hepatocytes are frequently used in drug metabolic pathways, metabolic velocity, the inhibition and induction of CYP450 enzyme and toxicity mechanism studies.20,21)
Similarly in liver microsomes, PXT was shown to inhibit MDZ 4-hydroxylation mediated by CYP3A but activate its 1′-hydroxylation after culturing for 45 min in primary rat hepatocytes. This phenomenon could be explained by the two-site model or multi-site model of CYP3A4 enzyme. The binding of PXT to one site caused an allosteric effect on the other MDZ-binding site or conformational transitions in CYP3A4 enzyme, leading to the different kinetics of MDZ metabolism. With the extension of culturing time, however, PXT presented a significant inhibition on both MDZ 4-hydroxylation and 1′-hydroxylation. Besides the competitive binding to CYP3A enzyme active center between PXT and MDZ, there is a potential of CYP3A expression downregulation after long-time (6 h) intervention of PXT.
To further study the impact of PXT on CYP3A1/2 mRNA expression, a RT-PCR experiment was investigated in primary rat hepatocytes after treatment with PXT and DEX. The result revealed that DEX weakly induced CYP3A1/2 mRNA expression following the incubation of 6 h, which was different from those reported in literatures.22) PXT and DEX didn’t affect CYP3A1/2 mRNA expression after culturing for 45 min. However, after incubation for 6 h in the presence of PXT (4.0, 8.0 µg/mL), CYP3A1 mRNA expression level was decreased to 87 and 80% of that in control, and CYP3A2 mRNA expression level was decreased to 89 and 85% of that in control, respectively. These results were consistent with the above inhibition effect of PXT on 4-hydroxylation and 1′-hydroxylation of MDZ mediated by CYP3A in rat hepatocytes after a long time intervention. PXT could inhibit CYP3A-mediated MDZ 4-hydroxylation and 1′-hydroxylation by down-regulating CYP3A1/2 mRNA expression, ultimately resulting in the reduction of CYP3A enzyme activity.
In summary, this research well explained the differential effect mechanism of PXT on MDZ metabolism from the perspective of enzyme kinetics using multiple-sites model in rat and human liver microsomes. The current study also deeply clarified a potential interaction mechanism that PXT could inhibit the enzyme activity by downregulating mRNA expression of CYP3A enzyme in primary rat hepatocytes after a long time intervention. PXT, as one of the important active components of Shenmai injection widely used for the treatment of cardiovascular disease, had the potential to cause drug–drug interactions mediated by CYP3A4 when co-administered with other clinical drugs. More systematic studies are needed to conduct about the effect of PXT on CYP3A4 enzyme in vivo in rats and human.
This work was supported by National Nature Science Fund (No. 81060275, No. 81160411), Jiangxi Province National Nature Science Fund (20151BAB205084), and Jiangxi Province Young Scientist Supporting Project (20133BCB23006).
The authors declare no conflict of interest.