2015 Volume 38 Issue 11 Pages 1722-1731
2015 Volume 38 Issue 11 Pages 1722-1731
α5β1 Integrin, a fibronectin receptor, is becoming a pertinent therapeutic target and a promising prognostic biomarker for cancer patients. The aim of this study was to functionalize an α5β1-specific fibronectin–mimetic peptide sequence KSSPHSRN(SG)5RGDSP (called PR_b) as a positron emission tomography (PET) probe. PR_b was modified by addition of a β-alanine residue, conjugated with 2-S-(4-isothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid (p-SCN-Bn-NOTA), and radiolabeled with 18F based on the chelation of 18F-aluminum fluoride. A control probe was produced by glycine to alanine substitution in the RGD motif of PR_b. Cell binding and blocking assays, autoradiographic evaluation of tissue binding and blocking, dynamic PET scans, and a biodistribution study were conducted using cell lines and murine tumor models with determined expression levels of α5β1 and other related integrins. 18F-PR_b was produced with a labeling yield of 22.3±1.9% based on 18F-F−, a radiochemical purity of >99%, and a specific activity of 30–70 GBq/µmol; it exhibited α5β1-binding activity and specificity in vitro, ex vivo, and in vivo, and had a rapid blood clearance and a predominant renal excretion pathway. In vivo α5β1-positive tumors could be clearly visualized by 18F-PR_b PET imaging. Both imaging and biodistribution studies suggested higher uptake of 18F-PR_b in α5β1-positive tumors than in α5β1-negative tumors and higher α5β1-positive tumor uptake of 18F-PR_b than the control probe. In contrast, there was no significant difference seen in the contralateral muscle uptake. A PET radioprobe, 18F-PR_b, was developed de novo and potentially can be used for noninvasive detection of α5β1 expression in tumors.
Integrins are a family of transmembrane glycoproteins consisting of 2 noncovalently associated α and β subunits. α5β1 Integrin (α5β1) mediates cell-extracellular matrix protein interactions through its major ligand, fibronectin. α5β1 is overexpressed in various cancers and in tumor vascular endothelium and plays important roles in tumor progression, angiogenesis, and metastasis.1) Suppression of α5β1 activities has been shown to potentiate the efficacy of chemo- or radiotherapy and induce apoptosis.2,3) Overexpression of α5β1 revealed that it had prognostic value for patients with non-small cell lung cancer,4) colorectal cancer,5) cervical cancer,6) and gastric cancer.7)
A number of α5β1 antagonists including antibodies, peptides, and small nonpeptidic molecules have been developed as therapeutic agents, and some are in clinical trials.1,8,9) However, there is still a lack of efficient molecular probes for noninvasive in vivo imaging of α5β1 expression, for use in evaluating malignancy, predicting prognoses, selecting subpopulations of patients who might be sensitive to α5β1-targeted drugs, assessing and monitoring of anti-tumor efficacy of α5β1-targeted drugs in clinical trials, and for contributing to the development of new drug therapies.
The efficient binding of α5β1 to its natural ligand, fibronectin, requires the involvement of 2 peptide sequences, Pro-His-Ser-Arg-Asn (PHSRN, the synergy binding site) and Arg-Gly-Asp (RGD, the primary binding site) on the 9th and 10th type-III repeats of fibronectin, respectively.10,11) PR_b, KSSPHSRN(SG)5RGDSP, is an α5β1-specific fibronectin–mimetic peptide developed by Kokkoli and colleagues.12) The presence of (SG)5 was designed to closely mimic both the distance and the hydrophobicity/hydrophilicity between PHSRN and RGD in native fibronectin, while the motif KSS served as a spacer for functional conjugation.12,13) The binding affinity of the PR_b ligand to the isolated α5β1 receptor was determined to have a dissociation constant, Kd, of 0.0763±0.0063 µM, which is strikingly stronger than that of the GRGDSP peptide (Kd, 270±353 µM), and only moderately weaker than that of the fibronectin (Kd, 0.03 µM).14) In terms of integrin specificity, studies performed by Kokkoli and colleagues demonstrate that as compared to simple RGD peptides, PR_b is more specific to α5β1 integrin.12,15) So far PR_b has been applied for the development of targeted drug-delivery systems using various nanoparticles.15,16)
Positron emission tomography (PET), with high sensitivity and spatial resolution, has become the predominant strategy for noninvasive visualization and quantification of molecular events in vivo, and fluorine-18 (18F; 109.8 min half-life) is the most commonly used radionuclide in clinical PET. Peptides are usually radiolabeled with 18F in a conventional multi-step and time-consuming procedure requiring synthesis and purification of an 18F-labeled synthon or prosthetic group. McBride et al. developed a novel and facile 18F-labeling method based on the chelation of the 18F-aluminum fluoride (Al18F) complex using a peptide-conjugated 1,4,7-triazacyclononane-triacetic acid (NOTA) ligand.17) The present study explored the development of PR_b as a potential 18F-labeled PET probe for noninvasively visualizing and quantifying α5β1 expression in vivo. PR_b was modified for conjugation with a bifunctional NOTA-based chelator and labeled using the Al18F method. The biological characteristics of 18F-labeled PR_b were evaluated in vitro, ex vivo, and in vivo.
The PR_b peptide, KSSPHSRN(SG)5RGDSP was synthesized by the standard 9-fluorenylmethoxycarbonyl (Fmoc)-based solid phase method using an Applied Biosystems 433 A peptide synthesizer. Deblocking of the protected peptide was carried out in a solution containing trifluoroacetic acid (TFA), thioanisole, ethanedithiol, phenol, and water at room temperature (RT) for 2 h. For 18F-labeling, one β-alanine was added to the N-terminus of PR_b to produce the sequence β-Ala-KSSPHSRN(SG)5RGDSP, which was then conjugated at the N-terminus with the chelating agent 2-S-(4-isothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid (p-SCN-Bn-NOTA, Macrocyclics Inc., Dallas, TX, U.S.A.). In brief, this peptide resin (0.1 mmol), dissolved in N,N-dimethylformamide (DMF, 5 mL) with triethylamine (10 µL), was reacted with p-SCN-Bn-NOTA (0.15 mmol) in DMF (1 mL) at RT for 12 h, and followed by the above-mentioned deblocking procedure. A control peptide β-Ala-KSSPHSRN(SG)5RADSP (β-Ala-PR_b control peptide) was synthesized and conjugated with p-SCN-Bn-NOTA in the same way. The crude products were purified by reversed-phase (RP)-HPLC and freeze dried using the following RP-HPLC conditions: solvent A=0.1% TFA in water, solvent B=0.1% TFA in acetonitrile, the flow rate=1.0 mL/min, the linear gradient=5 to 40% solvent B in 20 min, UV absorbance detection at 220 nm, and a SunFire C18 column (5 µm, 4.6×150 mm; Nihon Waters K.K., Tokyo, Japan). The purity of all products, PR_b, p-SCN-Bn-NOTA-conjugated β-Ala-PR_b [NOTA-PR_b], and p-SCN-Bn-NOTA-conjugated β-Ala-PR_b control peptide [NOTA-PR_b control], was >95%. Mass analysis (performed by an Applied Biosystems Voyager System 6366) showed the results: PR_b (calculated and observed molecular weight: 2145.1 and 2146.19, respectively); NOTA-PR_b (calculated: 2666.7; observed: 2666.64); NOTA-PR_b control (calculated: 2680.7; observed: 2680.83). All of the peptides were produced by Scrum Inc. (Tokyo, Japan).
Radiolabeling and StabilityNOTA-PR_b and NOTA-PR_b control were labeled with 18F based on the Al18F method.17) Ninety-five percent-enriched 18O-H2O was used for irradiation for the production of 18F by the 18O(p, n)18F nuclear reaction using a CYPRIS HM-18 cyclotron (Sumitomo Heavy Industries, Tokyo, Japan). 18F-Fluoride was purified by elution from a Sep-Pak Light QMA cartridge (Waters) with 0.5 mL of 0.4 M KHCO3, and a 200-µL fraction with an activity of 18F− (565–1669 MBq) was collected. The pH value of the 18F− solution was adjusted to 4 with 12 µL of metal-free glacial acetic acid. Ten microliters of 6 mM AlCl3 in sodium acetate buffer (0.1 M, pH 4.1) was then added and heated at 100°C for 15 min to form the Al18F complex. After cooling to RT, 200 µL of 1.87 mM NOTA-PR_b or NOTA-PR_b control dissolved in sodium acetate buffer was added and further heated at 100°C for 15 min for chelating of Al18F by NOTA. The reaction mixture was diluted with 200 µL of water–acetonitrile (9 : 1, v/v) containing 0.1% TFA and was purified with a semi-preparative RP-HPLC (solvent A=0.1% TFA in water, solvent B=0.1% TFA in acetonitrile, flow rate=5.0 mL/min, linear gradient=10 to 60% solvent B in 16 min, UV absorbance detection at 210 nm, and a Atlantis T3 column [10×150 mm; Nihon Waters K.K.]). The fraction containing the 18F-labeled peptide was collected and dried using a rotary evaporator. After reconstitution in normal saline (NS), purified 18F-PR_b was analyzed by RP-HPLC under the same conditions as described above except for the use of a 1 mL/min flow rate and a 4.6×150 mm Atlantis T3 column, and was analyzed again after 3 h of storage at RT to evaluate its stability.
In vitro plasma stability was examined by incubation of 10 µL 18F-PR_b mixed with 90 µL mouse plasma and 100 µL phosphate-buffered saline (PBS) at 37°C for 1 min and for 30 min. An equivalent quantity of 18F-PR_b was incubated in 190 µL PBS at 37°C for 1 h as a standard control. For in vivo blood stability, 18F-PR_b (ca. 11.1 MBq) was injected into normal mice via the tail vein, and at 10 and 30 min post-injection the mice were euthanized, and blood was collected and centrifuged at 2300×g for 5 min at 4°C. The plasma supernatants were collected with an extraction efficiency of >85%. All samples were directly analyzed by RP-HPLC without precipitating plasma proteins because we observed that, after protein precipitation with the addition of either ethanol or acetonitrile, the peptide tended to be retained on the column. The relative radioactivity of the radiolabeled peptide was expressed in millivolts (mV).
Cells and Tumor ModelsB16-F10 murine melanoma cells (high expression of α5β1 and minimal expression of αV integrin subunit)18) and SW48 human colorectal carcinoma cells (α5β1-, αVβ3- and αVβ5-negative)19) were obtained from the American Type Culture Collection (B16-F10: ATC C CRL-6475; SW48: ATC C CCL-231) and cultured according to their instructions. Animal procedures were approved by the Institutional Animal Care and Use Committee of the National Institute of Radiological Sciences (NIRS, Chiba, Japan). Four to six-week old female BALB/c-nu/nu athymic mice and C57BL/6 mice (the original source of the B16-F10 melanoma tumor cells) were obtained from CLEA Japan, Inc. (Tokyo, Japan). Tumor models were established by subcutaneous injection of 1×106 B16-F10 cells or 10×106 SW48 cells into the right shoulder of the mice, and the tumors were used for experiments after reaching 7–10 mm in diameter.
In Vitro α5β1 Expression and Binding AssayExpression status of α5β1 and its 2 subunits in B16-F10 and SW48 cells was validated by flow cytometric analysis. B16-F10 cells (1×106 cells) were incubated on ice for 30 min with primary rat anti-mouse monoclonal antibody to the integrin α5 subunit (rat IgG2a, clone 5H10-27, 1 : 100 dilution; BD Pharmingen, BD Biosciences), β1 subunit (rat IgG2a, clone KM16, 1 : 100 dilution; eBioscience, San Diego, CA, U.S.A.), or α5β1 (rat IgG2bk, clone BMA5, 1 : 100 dilution; Millipore, Temecula, CA, U.S.A.), monoclonal rat IgG2a (clone eBR2a, 1 : 100 dilution; eBioscience) or IgG2bk isotype control (clone eB149/10H5, 1 : 100 dilution; eBioscience), or antibody diluent only (PBS containing 1% bovine serum albumin (BSA) and 1 mM CaCl2). After washing with cold PBS, the cells were incubated on ice for 30 min with fluorescein-conjugated goat anti-rat immunoglobulin G (IgG) secondary antibody (1 : 100 dilution; Thermo Scientific, Rockford, IL, U.S.A.). The cells were washed, resuspended in PBS, and analyzed by flow cytometry using a Guava EasyCyte Plus System (Guava Technologies-Millipore, Hayward, CA, U.S.A.).
Similar procedures were performed to test the expression status of α5β1 in SW48 cells. The cells were directly stained with fluorescein-conjugated mouse anti-human α5 subunit antibody (clone SAM-1, 1 : 10 dilution; Millipore) or β1 subunit antibody (clone P4G11, 1 : 100 dilution; Millipore), or indirectly stained by incubation with different clones of mouse monoclonal antibodies against the human α5 subunit (clone SAM-1, 1 : 50 dilution; LifeSpan BioSciences, Seattle, CA, U.S.A.; clone P1D6, 1 : 100 dilution; Abcam, Cambridge, U.K.), or mouse anti-human α5β1 monoclonal antibody (clone HA5, 1 : 100 dilution; Millipore) and the secondary antibody fluorescein-conjugated goat anti-mouse IgG (1 : 100 dilution; Millipore). Cells treated in the absence of primary antibody or with mouse IgG1 isotype control (clone DAK-GO1, 1 : 100 dilution; Dako, Glostrup, Denmark) were used as negative controls.
In vitro cell-binding activities of 18F-PR_b were assessed according to our published method.20) In brief, trypsinized and resuspended cells were incubated with 18F-PR_b or 18F-PR_b control using various buffers, divalent cations, incubation time, temperature, or cell density conditions as indicated in Fig. 4. Competitive inhibition assays were performed by co-incubation of 18F-PR_b with increasing molar concentrations of unlabeled PR_b or NOTA-PR_b. The cell-bound radioactivity was measured using a gamma counter (1480 Wizard 3, PerkinElmer, Inc., Waltham, MA, U.S.A.) with decay correction, and the relative cell-binding ratio was defined as the percentage of the total added radioactivity that was cell-bound. IC50 values of PR_b and NOTA-PR_b were determined by nonlinear regression analysis.
Cell Adhesion AssayThe Innocyte Cell Adhesion Microplate Assay Kit (EMD Biosciences, La Jolla, CA, U.S.A.) was used to determine the effect of NOTA-PR_b on B16-F10 cells binding to fibronectin and performed according to the manufacturer’s instructions. In brief, B16-F10 cells suspended in the binding buffer (Hank’s Balanced Salt Solution [HBSS] with 25 mM N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid [HEPES], 0.1% BSA, and 1 mM CaCl2) at a density of 0.25×106 cells/mL were incubated with NOTA-PR_b and PR_b at the indicated molar concentrations, at 37°C in 5% CO2 for 20 min, followed by the addition of 100 µL of the reaction mixture each fibronectin-coated well and allowed to attach at 37°C in 5% CO2 for 1.5 h. After removal of the binding buffer and gentle washing with PBS, the fluorescent dye calcein-AM solution was added and incubated at 37°C in 5% CO2 for 1 h. The relative cell attachment was determined by measuring the fluorescence on a microplate reader at an excitation wavelength of 485 nm and an emission wavelength of 520 nm.
Ex Vitro α5β1 Expression and Binding AssayExpression of α5β1 in B16-F10 tumor, SW48 tumor, and murine kidney (the most common excretory organ for peptides) was examined by immunohistofluorescence staining. Serial frozen tissue sections (7 µm thick) of B16-F10 tumors and murine kidney were fixed in cold acetone, incubated with the primary rat anti-mouse α5β1 monoclonal antibody (1 : 100 dilution) or the monoclonal rat IgG2bk isotype control (1 : 100 dilution) at 4°C overnight, and labeled with the fluorescein-conjugated goat anti-rat secondary antibody (1 : 100 dilution) and incubated for 1 h at RT. For staining of the nucleus, slides were mounted with Dapi Fluoromount-G (SouthernBiotech, Birmingham, AL, U.S.A.) containing 4′,6-diamidino-2-phenylindole (DAPI). Fluorescence images (green, fluorescein; blue, DAPI) were acquired with an epifluorescence microscope (Olympus X61).
The expression status of α5β1 in SW48 tumor was evaluated using primary rabbit anti-human (with cross-reactivity to mouse) α5 polyclonal antibody (1 : 100 dilution; Novus Biologicals, Littleton, CO, U.S.A.), polyclonal rabbit IgG isotype control (1 : 250 dilution; Novus Biologicals), and fluorescein-conjugated goat anti-rabbit IgG secondary antibody (1 : 100 dilution; Santa Cruz Biotechnology, Heidelberg, Germany). B16-F10 tumor was used as a positive control.
Autoradiographic studies of 18F-PR_b tissue binding and blocking were performed based on our previously published procedure.21) In brief, 7-µm-thick frozen tissue sections were air-dried, blocked for nonspecific binding in HBSS with 25 mM HEPES and 0.1% BSA for 20 min, and incubated for 1 h in optimized binding buffer (determined by in vitro binding assays) containing 18F-PR_b (ca. 370 kBq). The blocking study was performed in serial sections by incubation of ca. 25 kBq 18F-PR_b or 18F-PR_b control with or without co-incubation of unlabeled PR_b at a concentration of 200 µM, a dose estimated from the results of an in vitro blocking assay. After incubation, the sections were autoradiographed on BAS-MS 2040 imaging plates (FUJIFILM, Tokyo, Japan) for 1 or 12 h and analyzed using a bioimaging analyzer system (FLA-7000; FUJIFILM). Photo-stimulated luminescence per unit area was measured for quantitative comparison of tissue-bound radioactivity.
PET Imaging and Biodistribution StudyAn Inveon small-animal PET system (Siemens, Knoxville, TN, U.S.A.) was used for dynamic scanning (30 scans of 1 min each) after injection of the imaging agents via tail vein. Three groups of mice were studied, including 2 groups of B16-F10 tumor-bearing mice administered with 18F-PR_b (8.3±2.2 MBq, n=12) and 18F-PR_b control (10±1.9 MBq, n=9), and another group of SW48 tumor-bearing mice administered with 18F-PR_b (8.6±2.6 MBq, n=5). All scanned mice were displayed as a three dimensional (3D) decay-corrected maximum-intensity-projection (MIP) image using the Inveon Research Workplace software (version 4.0; Siemens). The 3D volume of interest-derived percentage injected dose per gram (%ID/g) values were calculated as described previously.22) Images were reconstructed using a 3D maximum a posteriori (MAP) method (18 iterations with 16 subsets, β=0.2) without attenuation correction. Image analysis was performed using the ASIPro VM Micro PET Analysis software (Siemens). The total injected dose was calculated using a decay correction of the total activity present at the time of injection (t=0). For radioactivity quantification in the tumor, both kidneys, and urinary bladder, regions of interest (ROIs) encompassing the whole tissue area on each coronal slice were drawn manually, and all ROIs were linked to form a 3D volume of interest (VOI) using the 3D (VOI) dimensionality tool. For each VOI, the percentage of the total injected dose (%ID) was calculated to represent the total activity accumulated in the urinary bladder and both kidneys and the mean %ID/g was used to represent tumor uptake, assuming a tissue density of 1 g/mL. To estimate the radioactivity in the blood pool, a ROI with a fixed size of 0.1 cm2 was placed over the heart, and the radioactivity was quantified as the mean %ID/g. The mean %ID/g of the muscles contralateral to the tumors was also calculated.
Immediately after PET scan, the mice were euthanized, and their blood was drawn. Tumors and major organs were sampled and measured for radioactivity using a gamma counter with decay correction. Radioactivity was expressed as %ID/g normalized to a body weight of 20 g. It should be mentioned that the mice participated in this biodistribution study were randomly selected due to the convenience of experiment schedules.
Statistical AnalysisQuantitative data were expressed as mean±standard deviation (S.D.) and compared using a one-way ANOVA with the Dunnett’s multiple comparisons test. A p value <0.05 was considered significant.
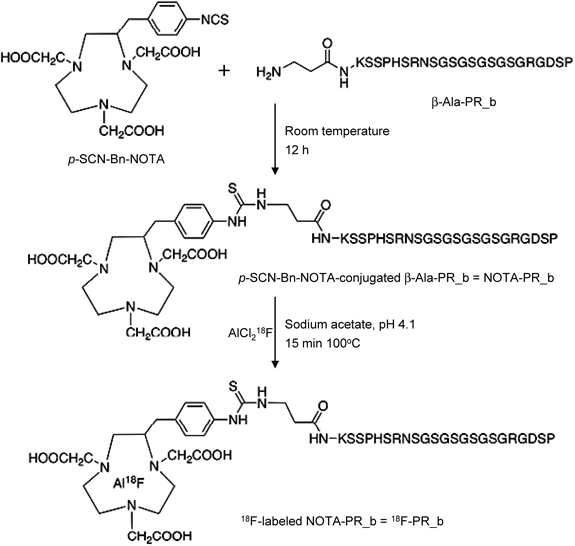
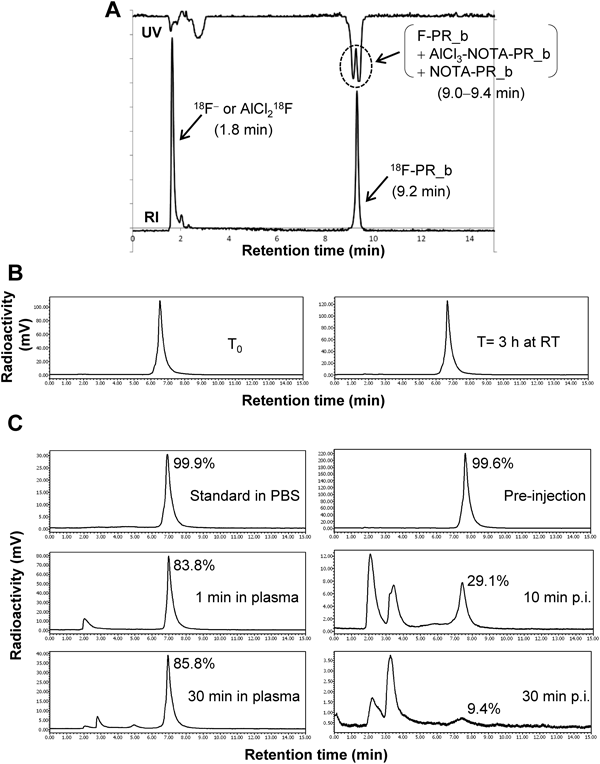
Peptide synthesis and radiolabeling procedures are schematically summarized in Fig. 1. RP-HPLC analysis and purification of the labeling mixture (Fig. 2A) demonstrated the presence of unbound 18F− or AlCl218F (retention time [tR]=1.8 min) and 18F-labeled PR_b (tR=9.2 min). Two UV peaks with tR 9.0–9.4 min revealed the mixture of labeled and unlabeled PR_b peptides as well as a possible complex of AlCl3-NOTA-PR_b. The process of radiolabeling, including the purification procedure, was completed within 1 h, with a decay-corrected labeling yield of 22.3±1.9% (n=7) based on 18F-F−. 18F-PR_b was obtained with >99% radiochemical purity and a specific activity of 30–70 GBq/µmol and was highly stable in NS, with >99% of 18F-PR_b remained intact for at least 3 h at RT (Fig. 2B). 18F-PR_b was found to be relatively stable in plasma in vitro (Fig. 2C, left panel). In vivo, the intact probe and a major fraction of the radioactive metabolites were detected in the plasma within 30 min post-injection (Fig. 2C, right panel), demonstrating that 18F-PR_b was metabolized rapidly, but detectable levels of intact probe remained.
Different sizes of sample loops, 321-µL (left panel) and 1-mL (right panel), were used for sample injection.
Flow cytometric analysis confirmed the positive expression of α5β1 in B16-F10 cells (Fig. 3A) and the negligible expression of α5β1 in SW48 cells (Fig. 3B).
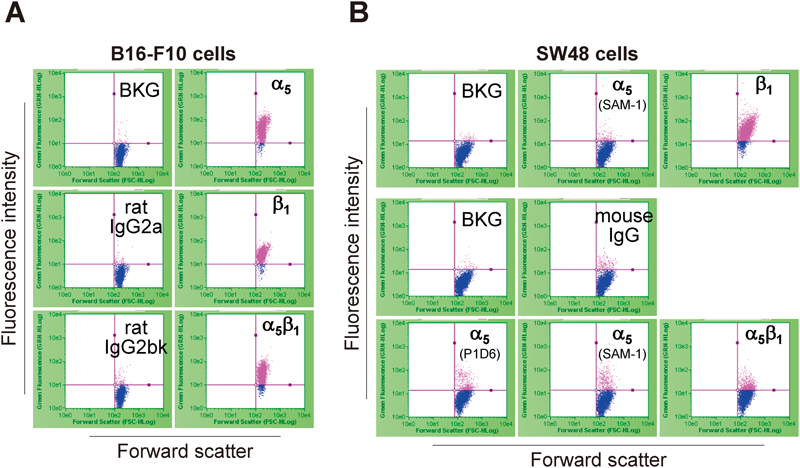
(A) Representative cytograms of B16-F10 cells incubated with rat anti-mouse α5, β1, or α5β1 monoclonal antibodies, antibody diluent (BKG), or rat IgG isotype control (IgG2a for α5 and β1; IgG2b for α5β1). (B) Representative cytograms of SW48 cells. Upper panel: Cells directly stained with fluorescein-conjugated mouse anti-human α5 or β1 monoclonal antibodies. Middle and lower panels: Cells incubated with mouse anti-human α5 (clone P1D6 or SAM-1) or α5β1 monoclonal antibodies, or mouse IgG isotype control.
The binding of 18F-PR_b to B16-F10 cells was influenced by the choice of incubation buffer, divalent cation composition, incubation time, and temperature (Figs. 4A–D). Based on these results and considering the relatively short half-life of 18F, the optimized binding conditions were: HBSS containing 25 mM HEPES, 0.1% BSA, and 1 mM CaCl2 as the binding buffer, and 1 h-incubation on ice, and were used for the subsequent in vitro assays unless otherwise stated.
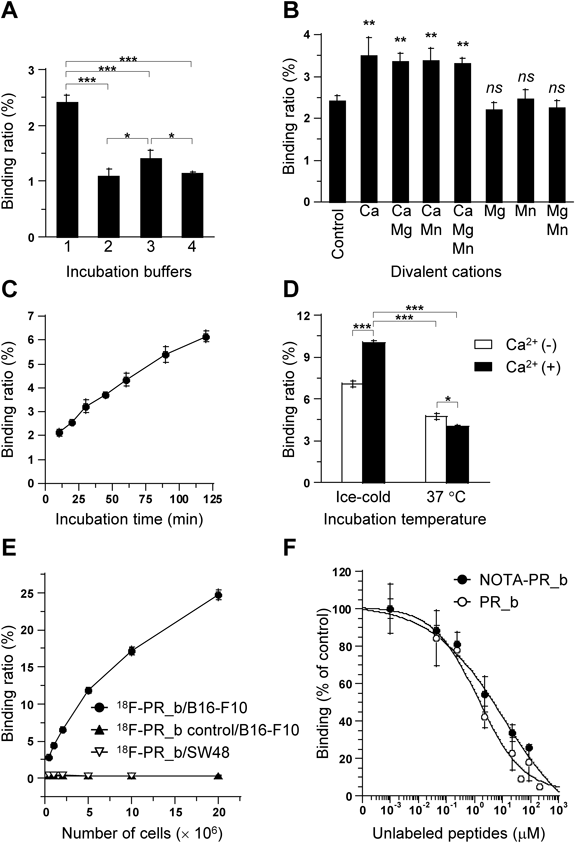
(A−D) B16-F10 cells (ca. 1×106 cells/1 mL) were incubated with 18F-PR_b (4.7 kBq [ca. 100000 cpm]) in (A) various buffers: (1) HBSS with 25 mM HEPES and 0.1% BSA, (2) PBS with 1% BSA, (3) PBS with 2% fetal bovine serum (FBS), or (4) Dulbecco’s modified Eagle’s medium with 2% FBS, on ice for 1 h (standard conditions unless otherwise stated); (B) buffer (1) in the absence (control) or presence of divalent cations: 1 mM Ca2+, Mg2+, or Mn2+, added individually or in combinations as indicated on the abscissa; (C) buffer (1) in the presence of Ca2+ for various time periods; (D) buffer (1) with or without Ca2+ on ice or at 37°C. (E) Comparative binding studies. Increasing numbers of B16-F10 or SW48 cells were incubated with 18F-PR_b or 18F-PR_b control (4.7 kBq) in buffer (1) with Ca2+ (binding buffer). (F) Competitive binding assays. B16-F10 cells (5×106 cells/mL) were incubated with 18F-PR_b (2.4 kBq) in the binding buffer in the absence (control) or presence of increasing concentrations of unlabeled NOTA-PR_b or PR_b. The binding assay (n=2–3) was performed on ice for 1 h unless stated. The data for (F) are means of the means of 2–3 independent experiments. * p<0.05; ** p<0.001; *** p<0.0001; ns: p>0.05 vs. control.
The relative cell binding ratios of 18F-PR_b to B16-F10 cells increased with increasing cell numbers, in contrast to the negligible binding of 18F-PR_b to SW48 cells and 18F-PR_b control to B16-F10 cells (Fig. 4E). Unlabeled PR_b and NOTA-PR_b inhibited the binding of 18F-PR_b to B16-F10 cells in a similarly concentration-dependent manner, with IC50 values of 1.9±1.1 µM and 4.8±2.3 µM, respectively (Fig. 4F).
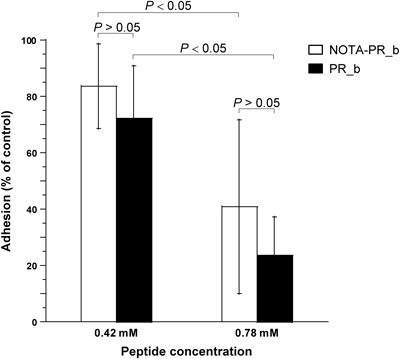
Effect of NOTA-PR_b versus PR_b on Cell Adhesion to FibronectinEffects of NOTA-PR_b and PR_b on α5β1-positive B16-F10 cell adhesion to fibronectin-coated surface were examined. NOTA-PR_b was found to be nearly as effective as PR_b for functionally blocking B16-F10 cell attachment to fibronectin (Fig. 5).
α5β1 Immunohistofluorescence showed positive staining in B16-F10 tumors developed in C57BL/6 and nude mice (Fig. 6A, upper panel) and negative staining in murine kidneys (Fig. 6A, upper panel) and SW48 tumors (Fig. 6A, lower panel).
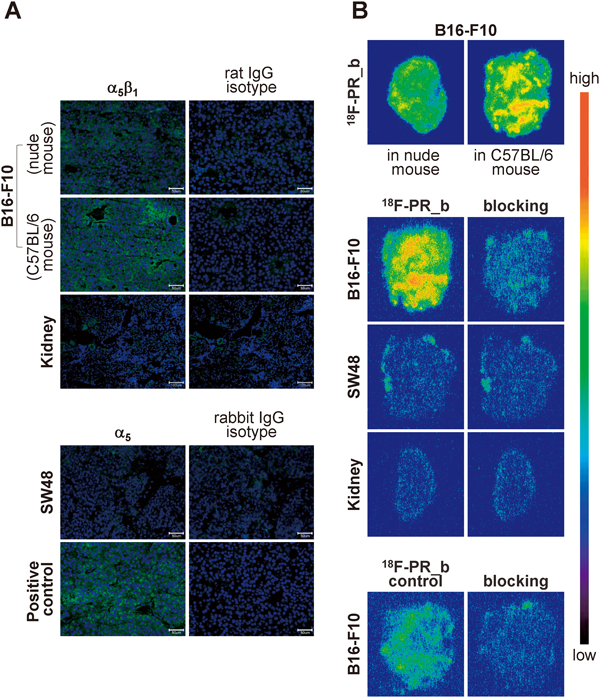
(A) Upper panel: Frozen sections of B16-F10 tumors in nude mice and in C57BL/6 mice, and murine kidney were incubated with rat anti-mouse α5β1 monoclonal antibody or rat IgG isotype control; Lower panel: Frozen sections of SW48 tumor were incubated with rabbit polyclonal antibody against human and mouse α5 or rabbit IgG isotype control. B16-F10 tumors were used as a positive control. Scale bar=50 µm (tumors) or 100 µm (kidneys). (B) Upper panel: Frozen sections of B16-F10 tumors in nude mice and in C57BL/6 mice were incubated with 18F-PR_b; Middle panel: Frozen sections of B16-F10 tumor (C57BL/6 mice), SW 48 tumor, and murine kidney were incubated with 18F-PR_b in the absence or presence (blocking) of an excess of unlabeled PR_b. Lower panel: B16-F10 tumor (C57BL/6 mice) sections were incubated with 18F-PR_b control in the absence or presence (blocking) of an excess of unlabeled PR_b.
Autoradiographic evaluation of frozen tissue sections after incubation with 18F-PR_b showed a higher degree of radioactive staining in B16-F10 tumors in C57BL/6 mice than that in nude mice (Fig. 6B, upper panel). A B16-F10 tumor model in C57BL/6 mice was used for the subsequent studies unless otherwise stated.
18F-PR_b binding properties were further investigated in serial sections of B16-F10 tumors, SW48 tumors, and murine kidney (Fig. 6B, middle panel). In contrast to the negligible radioactivity signals in SW48 tumors and kidney, strong radioactivity was observed in B16-F10 tumors, >90% of which could be blocked by unlabeled PR_b. As compared to the 18F-PR_b, 18F-PR_b control showed weaker binding to B16-F10 tumors, with ca. 80% reduction observed after blocking with unlabeled PR_b (Fig. 6B, lower panel).
PET and Biodistribution StudiesB16-F10 tumors were clearly visualized using 18F-PR_b with high contrast relative to the contralateral background at 25–30 min post-injection (Fig. 7A), in comparison to the weak radioactivity exhibited by the 18F-PR_b control in the peripheral region of B16-F10 tumors (Fig. 7B) and the negligible radioactivity of 18F-PR_b in SW48 tumors (Fig. 7C).
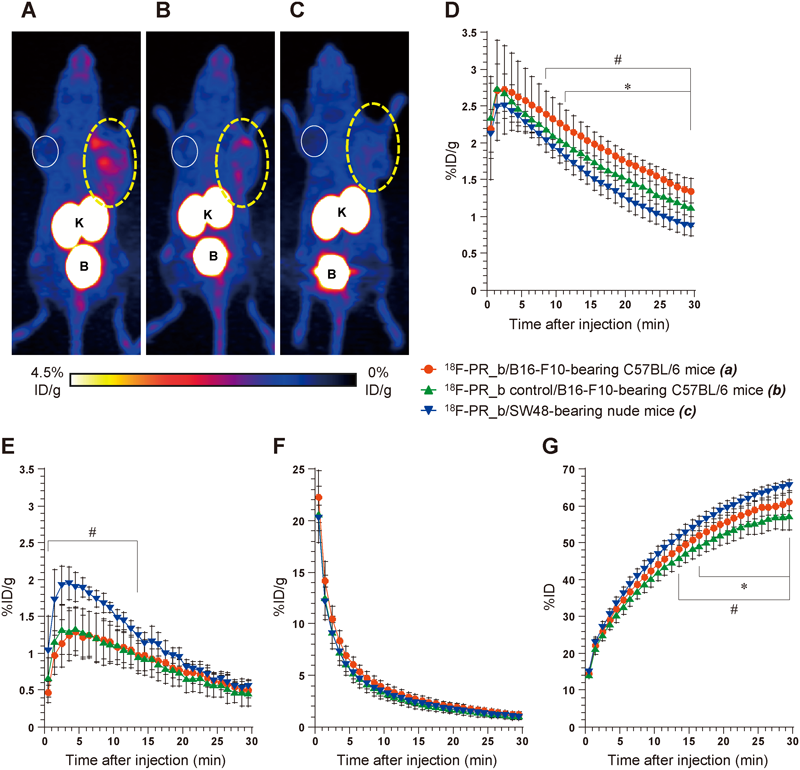
Dotted and solid circles indicate tumor and contralateral muscle locations, respectively. K=kidneys; B=urinary bladder. (a) vs. (b): * p<0.05; (a) vs. (c): #p<0.05.
As shown in Fig. 7D, 18F-PR_b exhibited significantly higher uptake in B16-F10 tumors than in SW48 tumors (1.35±0.16 vs. 0.87±0.13%ID/g, respectively, at 30 min post-injection, p=0.0002) and significantly higher uptake in B16-F10 tumors compared to its control probe (1.35±0.16 vs. 1.12±0.25%ID/g at 30 min post-injection, p=0.023). In contrast, the contralateral muscle uptake did not show significant difference between 18F-PR_b and its control probe during the 30-min scanning period and between C57BL/6 and nude mice from 14 to 30 min post-injection (Fig. 7E). Rapid blood clearance of radioactivity was observed for all the mice evaluated, with no significant difference between 18F-PR_b and its control probe and between C57BL/6 and nude mice (Fig. 7F). Time–activity curves for the total activities of both kidneys plus bladder (Fig. 7G) demonstrated rapid and steady excretion of the radiolabeled peptides by the renal system within 30 min after injection, and showed some difference between the mouse strains and between the different probes.
As shown in Table 1, the ex vivo biodistribution study performed following PET imaging demonstrates tissue distribution profiles of 18F-PR_b and its control probe and confirms the results of PET quantification.
| Organ | 18F-PR_b | 18F-PR_b control | 18F-PR_b |
|---|---|---|---|
| (B16-F10) | (B16-F10) | (SW48) | |
| Blood | 1.82±0.26 | 1.61±0.43 | 1.49±0.42 |
| Tumor | 1.07±0.14 | 0.88±0.23* | 0.79±0.08* |
| Muscle | 0.36±0.03 | 0.37±0.13 | 0.43±0.21 |
| Bone | 0.72±0.12 | 0.67±0.24 | 0.68±0.36 |
| Brain | 0.05±0.003 | 0.04±0.005* | 0.04±0.01 |
| Heart | 0.61±0.02 | 0.47±0.11* | 0.49±0.04 |
| Lung | 1.18±0.08 | 0.78±0.08* | 1.04±0.20 |
| Liver | 0.48±0.04 | 0.30±0.05* | 0.39±0.07 |
| Pancreas | 0.40±0.05 | 0.33±0.02* | 0.26±0.01* |
| Spleen | 0.41±0.02 | 0.34±0.04* | 0.36±0.04 |
| Stomach | 0.82±0.34 | 0.25±0.04* | 0.47±0.11 |
| Small intestine | 0.63±0.04 | 0.45±0.05 | 0.65±0.36 |
| Large intestine | 0.50±0.06 | 0.52±0.03 | 0.31±0.08 |
| Kidney | 73.4±0.62 | 63.8±6.5 | 62.3±15.8 |
| Skin | 1.43±0.30 | 1.04±0.27 | 0.90±0.27 |
Tissue radioactivity was assessed immediately after the PET scan, i.e., at 30 min post-injection of 18F-PR_b or 18F-PR_b control and is expressed as %ID/g (mean±S.D.). n=6–10/each group for blood and tumor and n=3–4/each group for other organs; * p< 0.05 vs. group of B16-F10-bearing mice that received 18F-PR_b.
There are a few reports on the development of α5β1-specific radiotracers.23) Neubauer et al.24) recently reported on a 68Ga-labeled radiotracer based on an aza-glycine derivative of an α5β1 antagonist that was initially characterized by Heckmann et al. based on a nonpeptidic tyrosine scaffold.25) PET imaging of mice bearing an α5β1-positive tumor and an αVβ3-positive tumor on each side of the shoulder demonstrated promising results of this peptidomimetic radiotracer for selective imaging of α5β1. Koivunen et al.26) isolated a lead peptide GACRRETAWACG A from a phage display library with a high specificity and affinity for α5β1. This peptide was recently radiolabeled with 18F, and it was found to be stable in human serum.23) However, biodistribution studies demonstrated this radiotracer was not suitable for in vivo imaging due to its considerably high and constant radioactivity accumulation in the blood and other major organs.23) In contrast to the above-mentioned approaches, PR_b was developed to mimic the natural states of 2 α5β1-binding regions PHSRN and RGD in fibronectin. To the best of our knowledge, the present study is the first to explore the possible functionalization of α5β1-specific fibronectin–mimetic peptide for in vivo imaging of α5β1 expression, and encompasses the design, synthesis, radiolabeling, stability examination, and in vitro, ex vivo, and in vivo bioactivity studies.
Several peptide ligands, including an octreotide analog, a bombesin peptide, and cyclic RGD-containing peptides, have been radiolabeled with 18F based on the Al18F method.27) Our study shows that the fibronectin–mimetic peptide PR_b could also be similarly radiolabeled with 18F with a labeling yield of 22.3±1.9%, which is comparable to the labeling yield of 17.9% reported for 18F-AlF-labeled NOTA-conjugated cyclic RGD dimer.28) However, this technique would benefit from a higher labeling yield and a decreased amount of peptide required for labeling. Future work should be focused on identifying and evaluating the factors that influence radiofluorination, such as the reaction buffer, the balance between the amounts of 18F− and Al3+, the pH value, and the chelator.
Integrins, although not fully understood, are known to exist in inactive and active conformational states, which can be modulated by external stimuli such as divalent cations.20,29,30) Imaging using atomic force microscopy has shown that Mn2+ increased the adhesion of immobilized α5β1 to the GRGDSP ligand, and the addition of Ca2+ compromises the effect of Mn2+.31) In contrast, we observed Ca2+-dependent binding of PR_b to α5β1-positive B16-F10 cells, which was not influenced by the addition of either Mg2+ or Mn2+. These differences may be due to the different states of α5β1 (the isolated α5β1 versus that existing in cells) as well as the different bioevaluation systems. Besides the divalent cations, the binding activities of 18F-PR_b to B16-F10 cells was also influenced by the choice of incubation buffer, incubation time, and temperature, and the involved mechanisms should be clarified in future study.
The α5β1 integrin specificity of PR_b has been extensively studied and validated by Kokkoli and colleagues using blocking studies with specific anti-integrin antibodies and peptides and using scrambled peptide sequences,12,31) and further confirmed in α5β1-targeted drug delivery studies.16,32,33) As mentioned, the α5β1-binding affinity of PR_b is only moderately weaker than the native ligand fibronectin. PR_b is hence distinguished from other simple linear RGD peptides that may also bind to several αV integrins, such as αVβ3 and αVβ5, with very low binding affinity.34–36) For example, our previous study20) demonstrated that a linear fibronectin-derived monomeric AVTGRGDSY peptide, labeled with either 125I or a fluorescent dye Cy5.5, bound in a negligible level to αVβ3-overexpressing HEK293(β3) cells under the similar cell-binding assay conditions used for evaluation of 18F-PR_b. In the present work, under in vitro conditions, the α5β1 specificity of 18F-labeled PR_b was supported by comparison of (1) the binding of 18F-PR_b to α5β1-positive B16-F10 and α5β1-negative SW48 cells and (2) the cell-binding activities between 18F-PR_b and its control probe. It should be mentioned that B16-F10 cells had minimal expression of αV integrin subunit, and SW48 cells did not express both αVβ3 and αVβ5. Such two cell lines were used in the present study for excluding the influence of other RGD-recognizing integrins, especially αVβ3 and αVβ5. In addition, binding assays using αVβ3-overexpressing HEK293(β3) demonstrate that 18F-PR_b had almost negligible binding activity to αVβ3 integrin (Supplementary Fig. 1). The α5β1-binding specificity of 18F-PR_b was further confirmed by the dose-dependent inhibition of the binding of 18F-PR_b to α5β1-positive cells in the presence of unlabeled PR_b or NOTA-PR_b. The introduction of the p-SCN-Bn-NOTA-β-Ala moiety to PR_b was considered to not markedly compromise the receptor binding, because PR_b and NOTA-PR_b showed similar inhibitory effects on the binding of 18F-PR_b to α5β1-positive cells and on the adhesion of α5β1-positive cells to fibronectin-coated wells.
Ex vivo autoradiographic studies in frozen tissue sections demonstrated similar results as those obtained under in vitro conditions. 18F-PR_b showed a much higher degree of radioactive staining in α5β1-positive B16-F10 tumor than in α5β1-negative SW48 tumor. The radioactive staining produced by 18F-PR_b was also found weak in our previously reported αVβ1-overexpressing HEK293(β1) and αVβ3-overexpressing HEK293(β3) tumor models20,37) (data not shown), indicating a low binding affinity of 18F-PR_b for the αVβ1 and αVβ3 integrins. The selective binding of 18F-PR_b to α5β1-positive tumors was further supported by the blocking effect of an excess of unlabeled PR_b. Under ex vivo conditions, the 18F-PR_b control probe showed weak, but specific binding to α5β1-positive tumors. This could be attributed to the receptor-binding activity of the PHSRN motif alone because, in the control probe, the primary binding motif RGD was replaced by RAD, which abolished integrin-binding activity. Feng et al. reported that PHSRN specifically bound to α5β1 but with lower affinity than did RGD.38)
In vivo, α5β1-positive tumors were visualized using PET in mice injected with 18F-PR_b, and the binding specificity was demonstrated by paralleled comparative studies using α5β1-negative tumors and the RAD-containing control probe. It should be emphasized that RAD probes have been conventionally used as an in vivo control for the corresponding RGD probes because they have similar structural properties (differing only by a single methyl group), but possess discretely different binding abilities for integrin.37, 39) 18F-PR_b PET and biodistribution studies did not show obvious radioactivity accumulation in bone and other major normal organs, except the kidneys and urinary bladder, with no obvious sign of bone radioactivity accretion. Since 18F alone or non-chelated Al18F showed considerably high uptake in bone,17) the above results suggest that, under in vivo conditions, Al18F remained predominantly chelated to NOTA, which could be conjugated with the intact peptide or intermediate metabolites, with no significant detachment of 18F from NOTA. Stability studies (Fig. 2C) demonstrated that 18F-PR_b was relatively stable in mouse blood plasma, but rapidly metabolized in vivo within 30 min post-injection. The rapid blood clearance and relatively low metabolic stability of 18F-PR_b, which could be attributed to the linear structure of the peptide as observed for other linear peptide-based radiotracers,40) possibly compromised the efficient accumulation of the probe in the tumor. That is maybe why the differences in tumor uptake between 18F-PR_b and its control probe were not as large as the differences in cell- and tissue-bound activities between the two probes obtained from in vitro and ex vivo studies. The high kidney uptake is considered to be primarily due to the predominant renal excretion of 18F-PR_b and its radioactive metabolites because neither the binding of 18F-PR_b nor the expression of α5β1 by renal tissues was detected by ex vivo autoradiography and immunohistofluorescence staining. Finally, it is necessary to mention that in addition to our extensive discussion of the specificity of 18F-PR_b for the α5β1 in the present study, a series of blocking studies after further optimization of this probe in terms of metabolic stability, pharmacokinetics, and α5β1-binding affinity would further demonstrate the specificity of the probe.
In summary, the α5β1-specific fibronectin–mimetic peptide PR_b was successfully radiolabeled with 18F based on the facile Al18F method, and investigated in vitro, ex vivo, and in vivo. 18F-PR_b exhibited its potential for use as an imaging probe for α5β1 positive tumor detection, and is worth further investigation to optimize radiolabeling and to improve its binding activity and pharmacokinetic properties by peptide modification strategies such as multimerization and PEGylation.
This work was supported, in part, by a Grant-in-Aid for Scientific Research (C) 24591827 from the Ministry of Education, Culture, Sports, Science and Technology of Japan. We would like to thank the Molecular Probe Program for supplying the 18F produced for this study; the Cyclotron Operation Section for cyclotron operation; and all other members of Molecular Imaging Center, NIRS, for kindly assisting with the animal experiments.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.