2015 Volume 38 Issue 12 Pages 1836-1842
2015 Volume 38 Issue 12 Pages 1836-1842
Pyrrole–imidazole (PI) polyamide is a novel gene regulating agent that competitively inhibits transcription factor binding to the promoter of the specific target gene. Liver fibrosis is an integral stage in the development of chronic liver disease, and transforming growth factor β (TGFβ) is known to play a central role in the progression of this entity. The aim of this study was to evaluate the effect of PI polyamide targeting TGFβ1 on rat liver fibrosis. PI polyamide was designed to inhibit activator protein 1 (AP-1) transcription factor binding to the TGFβ1 gene promoter. The effect of PI polyamide on hepatic stellate cells was evaluated by real time polymerase chain reaction (PCR) in RI-T cells. To determine the effect of PI polyamide in vivo, PI polyamide was intravenously administered at a dose of 3 mg/kg/week in dimethylnitrosamine (DMN)-induced rat model of liver fibrosis. Treatment of RI-T cells with 1.0 µM PI polyamide targeting TGFβ1 significantly inhibited TGFβ1 mRNA expression. Azan staining showed that DMN treatment significantly increased areas of fibrous materials compared with controls. PI polyamide targeting TGFβ1 significantly decreased the fibrous area compared with DMN group. mRNA expression levels of α-smooth muscle actin and matrix metalloproteinase-2 were significantly increased in DMN-treated group compared with control. Treatment with TGFβ1 PI polyamide significantly decreased mRNA expression of these genes compared with DMN group. The novel gene regulator PI polyamide targeting TGFβ1 may be a feasible therapeutic agent for the treatment of chronic liver disease.
Liver cirrhosis is the end stage of all chronic liver diseases. Hepatic fibrosis is an integral phase in the development of chronic liver disease, and often precedes liver cirrhosis. Hepatic fibrosis is the common pathological basis of numerous chronic liver diseases including viral liver disease, alcoholic, non-alcoholic steatohepatitis, autoimmune hepatitis, primary biliary cirrhosis, and metabolic disease. Liver cirrhosis is characterized by the increase and excessive deposition of the liver extracellular matrix (ECM).1–3)
Excessive activation of transforming growth factor β (TGFβ) increases the synthesis and decreases the degradation of ECM proteins with a gradual destruction of organ tissue and structure.4–8) The major source of ECM deposition in the liver is hepatic stellate cells (HSCs). After liver injury, TGFβ promotes the activation and proliferation of hepatic HSCs.2,3,9–11) High levels of TGFβ1 are often found in liver fibrosis and there may be a positive correlation between the elevation of TGFβ1 mRNA level and fibrogenic activity.12–14) Thus, synthesis of ECM proteins increases in the liver due to excessive activation of the TGFβ signal transduction pathway. Therefore, this pathway has become a potential target for the treatment of the liver fibrosis.
Pyrrole–imidazole polyamide (PI polyamide) is a novel gene regulator composed of N-methylpyrrole (Py) and N-methylimidazole (Im). PI polyamide can inhibit DNA–protein interaction by binding to the minor groove of double-strand DNA with high affinity and sequence specificity. Antiparallel pairing of imidazole with pyrrole (Im/Py) recognizes G-C base pair, whereas a Py/Py pair recognizes either an A-T or T-A base pair. Interference of DNA-transcription factor protein interaction by PI polyamides inhibits the regulation of the gene transcription and modulates target protein expression.15–18) Various types of sequence specific PI polyamides have been developed to control gene expression.19–25) In a previous study, we designed PI polyamide targeting the consensus sequence of the activator protein 1 (AP-1) binding site on the TGFβ1 promoter and demonstrated that this had an anti-fibrogenic effect in the rat model of renal fibrosis.20,21)
This study was undertaken to evaluate the effect of PI polyamide targeting TGFβ1 in inhibiting liver fibrosis in the rat model of chronic liver disease.
PI polyamide targeting TGFβ1 was designed to span the boundary of the AP1 binding site of the TGFβ1 promoter. PI Polyamides were synthesized according to previously described methods.26,27) A mismatch polyamide that did not bind to the transcription binding sites of TGFβ1 promoter was also designed and synthesized.
Determination of mRNA ExpressionFor in vitro experiments, RI-T cells (JCRB1088) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum (FCS). After a 24-h culture with DMEM containing 0.5%FCS, the cells were incubated with 1.0, 0.1 or 0.01 µM PI polyamide targeting the TGFβ1 gene or 1.0 µM mismatch PI polyamide in DMEM with 0.5% FCS for 8 h. Four hours after the initiation of PI polyamide treatment, 0.01 µM phorbol-12-myristate-13 acetate (PMA) was added into medium to stimulate TGFβ expression. Six hours after PMA stimulation, total RNA was isolated and reverse-transcribed to cDNA as described previously.28) Real-time quantitative polymerase chain reaction (RTq-PCR) was performed with the cDNA (diluted 4 times) using TaqMan Universal Master Mix (Applied Biosystems, Foster City, CA, U.S.A.) and an ABI 7500 real-time PCR system (Applied Biosystems) according to the manufacturer’s instructions. Assay-on-Demand primers and probes were purchased from Applied Biosystems (TGFβ1, Rn01475963). 18S ribosomal RNA (rRNA) expression (Applied Biosystems 4319413E) was quantified for normalization of mRNA expression level among samples. RT-PCR data were analyzed using a standard curve. In all cases, the correlation coefficients for the standard curves were >0.90.
For the in vivo experiments, total RNA was isolated from 20 mg of liver tissue of a Sprague-Dawley rat and reverse-transcribed. RTq-PCR was performed with the cDNA. Assay-on-Demand primers and probes were purchased from Applied Biosystems (α-smooth muscle actin: Rn01759928_g1, collagen type IV: Rn01482927, and TGFβ1: Rn01475963).
In Vivo ExperimentsThis study conformed to the guidelines published in the Guide for the Care and Use of Laboratory Animals of the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996) and approved by Nihon University Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (7-weeks old; CLEA Japan, Tokyo, Japan) were used in all of the experiments. Rats were fed normal chow diet (Oriental Yeast, Tokyo, Japan) ad libitum for 2 weeks.
Biochemical ExaminationsSerum levels of total protein, albumin, total bilirubin, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured by the spectrophotometric method.
Definition of liver fibrosis was based on the relevant literature.29) Liver fibrosis was induced by intraperitoneal injections of dimethylnitrosamine (DMN; Sigma, MO, U.S.A.) on 3 consecutive days a week for 3 weeks. PI polyamide (1 mg/kg body weight) targeting TGFβ1 or mismatch polyamide was dissolved in 100 µL of 0.1% acetic acid and injected via the tail vein weekly for 4 weeks (Fig. 1).

Twenty-four rats were randomly divided into 4 groups: (1) normal control group (n=6, without DMN), treated with 0.1% acetic acid), (2) DMN group (n=6, treated with 1 µL of 0.5% DMN/kg, and 0.1% acetic acid), (3) mismatch group (n=6, treated with 1 µL of 0.5% DMN/kg, and 1 mg/kg mismatch PI polyamide in 0.1% acetic acid), (4) PI Polyamide group (n=6, treated with 1 µL of 0.5% DMN/kg, and 1 mg/kg PI polyamide targeting TGFβ1 in 0.1% acetic acid).
The rats were euthanized by a lethal injection of sodium pentobarbital (IP, 100 mg/kg body weight) 4 weeks after PI polyamide administration.
Histological Examination and Immunohistological StainingLiver tissue sections were stained with haematoxylin–eosin (HE) for histopathological examination and Azan staining was used for determination collagen area. The areas of collagen stained blue with Azan were quantitated using the image J software (NIH, Bethesda, MD, U.S.A.)30) by analyzing 15 random fields per slide and calculating the ratio of connective tissue to the whole area of the liver tissue.
Immunohistochemical staining was carried out to detect the expression of various marker proteins using mouse primary antibodies against α-smooth muscle actin (α-SMA) (1 : 100; M0851, DAKO, Glostrup, Denmark), collagen type IV (1 : 100; ab6586, Abcam, Cambridge, U.K.) and TGFβ (1 : 100; sc-146, Santa Cruz Biotechnology, Carlsbad, CA, U.S.A.) and horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) was used as the secondary antibody in each case.
Statistical AnalysisResults are given as the mean±standard error of mean (S.E.M.). The statistical significance of differences between mean values was evaluated by Student’s t-test for unpaired data. A p value ≤0.05 was considered statistically significant.
Treatment of RI-T cells with 0.1 µM PMA increased the TGFβ1 mRNA expression level by about 2.6 fold. When 1.0 µM PI polyamide was added, the TGFβ1 mRNA expression level was significantly decreased by about 0.6 fold compared with the control cells. Cells treated with 0.01 and 0.1 µM PI polyamide or 1 µM mismatch PI polyamide did not show a significant difference in the TGFβ1 mRNA expression level compared with the non treated cells (Fig. 2).
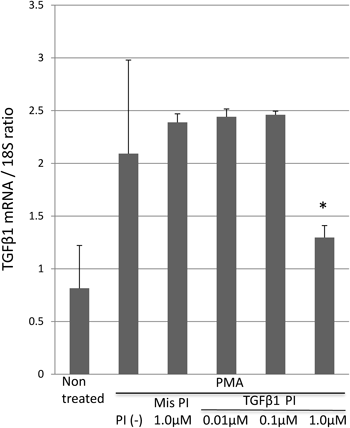
* p<0.05 vs. PMA(+)PI(−) group. PMA: phorbol-12-myristate-13 acetate, PI: PI polyamide, Mis: mismatch.
Intravenous administration of PI polyamide or mismatch did not affect the body weight and organ weights of Sprague-Dawley rat. There were no significant changes between the serum values of total protein, albumin, total bilirubin ALT and AST of DMN treated rats and PI polyamide treated rats. Serum ALT levels in DMN treated group, mismatch PI polyamide group and TGFβ1 PI polyamide group were significantly higher compared with that in control group (Table 1).
| Body weight (g) | Liver weight (%) | Spleen weight (%) | Total protein (g/dL) | Albumin (g/dL) | |
|---|---|---|---|---|---|
| A. Control | 504.2±69.2 | 41.8±4.2 | 2.3±0.8 | 6.4±0.5 | 4.1±0.1 |
| B. DMN | 489.0±20.2 | 44.1±8.8 | 3.1±0.6 | 6.1±0.2 | 4.1±0.2 |
| C. DMN-mismatch PI | 406.0±17.7 | 40.3±5.1 | 3.3±0.6 | 6.0±0.5 | 3.9±0.2 |
| D. DMN-TGF PI | 442.3±24.7 | 40.9±0.7 | 3.4±0.7 | 5.6±0.3 | 3.7±0.3 |
| Total bilirubin (mg/dL) | AST (IU/L) | ALT (IU/L) | |||
| A. Control | 0.05±0.01 | 123.0±19.7 | 41.4±8.1 | ||
| B. DMN | 0.06±0.01 | 165.3±79.3 | 53.8±2.9* | ||
| C. DMN-mismatch PI | 0.07±0.03 | 144.8±26.9 | 60.6±12.9* | ||
| D. DMN-TGF PI | 0.06±0.01 | 159.8±31.1 | 51.5±2.5* |
* p<0.05 vs. Control. Liver weight: Liver weight/body weight (%). Spleen weight: Spleen weight/body weight (%).
In this rat model, hepatic fibrosis was induced by DMN treatment. HE and Azan staining were performed to determine whether the extent of the DMN-induced liver damage was reduced by PI polyamide targeting TGFβ1.
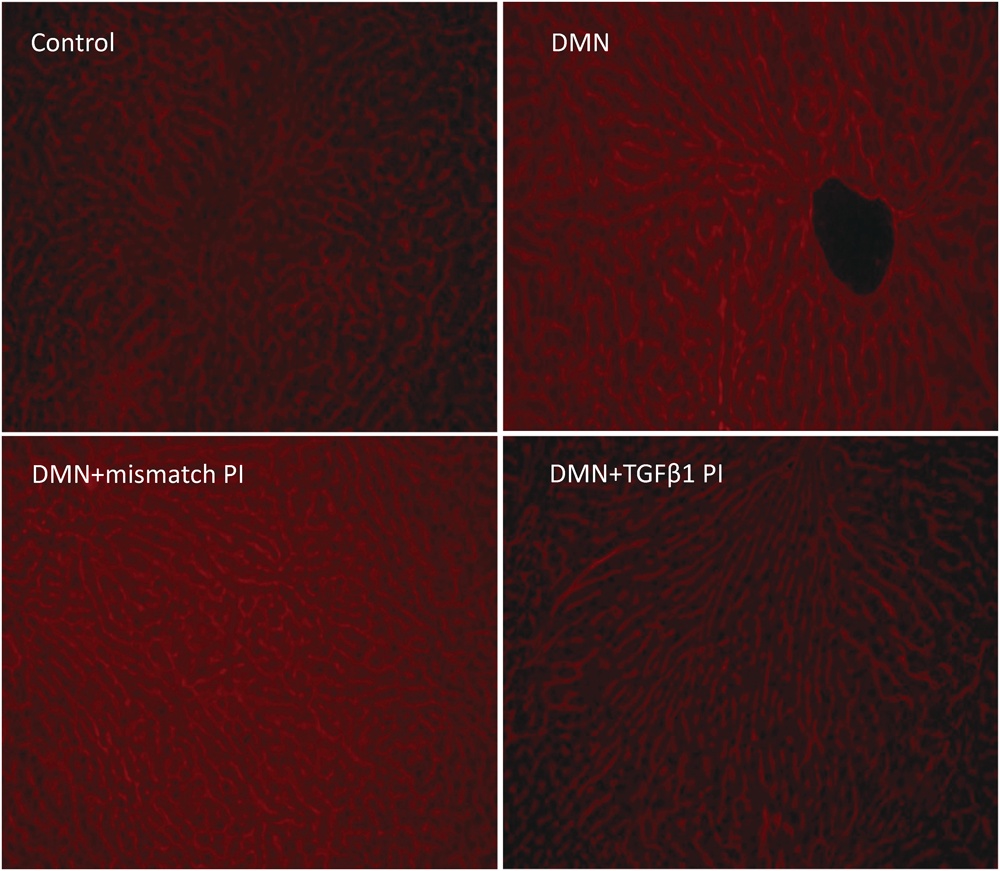
The livers of the control group rats showed normal lobular architecture and no fibrotic space. However, the DMN group showed dramatic changes consisting of the destruction of normal liver lobular architecture, bridging necrosis, inflammatory cell infiltration, fibrotic septae and pseudolobule formation. PI polyamide treatment suppressed these DMN-induced fibrosis including bridging necrosis, inflammatory cell infiltration, fibrotic septae and pseudolobule formation (Fig. 3). Figure 4 shows typical Azan staining results in which fibrous materials are stained blue. Little or no blue staining was observed in the controls. In contrast, the livers injured by DMN treatment displayed considerable accumulation of fibrous materials. DMN treatment for 3 weeks caused excessive deposition of collagen fibrils that was sufficient for the evaluation of the antifibrogenic effect of PI polyamide targeting TGFβ1. Based on the result of Azan staining, PI polyamide treatment clearly suppressed collagen accumulation. To quantitatively evaluate the effect of PI polyamide on fibrogenesis, we measured areas of fibrous materials stained blue using the Image-J software. DMN treatment significantly increased areas of fibrous materials compared with that in controls. PI polyamide targeting TGFβ1 significantly suppressed the fibrous area compared with that in DMN group (Fig. 5).

DMN: dimethylnitrosamine, PI: PI polyamide.

DMN: dimethylnitrosamine, PI: PI polyamide.

* p<0.05 vs. DMN group. DMN: dimethylnitrosamine, PI: PI polyamide.
The immunostaining experiments using α-SMA and collagen type IV antibody was performed to evaluate the effect of PI polyamide targeting TGFβ1 on liver fibrosis. Immunohistochemical result showed that DMN-induced accumulation of α-SMA, which was stained brown, was decreased by intravenous administration of 3 mg/kg of PI polyamide targeting TGFβ1 (Fig. 6). DMN-induced accumulation of collagen type IV, which was stained red, was also decreased by the administration of PI polyamide targeting TGFβ1 (Fig. 7).

DMN: dimethylnitrosamine, PI: PI polyamide.
DMN: dimethylnitrosamine, PI: PI polyamide (immunohistochemical staining).
The mRNA expression levels of α-SMA, collagen type Iα1, collagen type IV, matrix metalloproteinase-2 (MMP2), TGFβ1, and Tissue inhibitor of metalloproteinase-1 (TIMP1) were significantly increased in DMN treated group compared with that in control group. Treatment with TGFβ1 PI polyamide significantly decreased αSMA and MMP2 compared with that in DMN groups. Collagen type Iα1, collagen type IV, TGFβ1, and TIMP1 mRNA levels were lower in TGFβ1 PI polyamide group compared with that in DMN group, but they were not statistically significantly different from control (Fig. 8).

* p<0.05 vs. PI(−) group.
In the present study, the PI polyamide targeting the TGFβ1 gene promoter significantly inhibited the expression of TGFβ1 mRNA in cultured rat HSCs stimulated with PMA, suggesting this polyamide has the potential to control TGFβ1 gene expression. The DMN-induced rat model of liver fibrosis was suitable to test this effect in vivo. Administration of PI polyamide targeting the TGFβ1 promoter reduced the DMN-induced liver fibrosis without any significant adverse effects. PI polyamide targeting the TGFβ1 promoter might be a novel promising therapeutic agent for treatment of progressive liver fibrosis in chronic liver diseases.
Hepatic fibrosis is characterized by scaring due to chronic inflammation from liver disease. Liver fibrosis is a complicated pathological process in which multiple components including HSCs, Kupffer cells, hepatocytes, various cytokines, and ECM proteins are involved. Among those, activated HSCs are the major producers of fibrotic matrix.31,32) After liver injury, they become activated, developing a myofibroblast-like phenotype associated with increased proliferation and collagen synthesis. TGFβ1 is known to be the most potent fibrogenic cytokine that mediates HSC activation.33) Moreover, activated HSCs secrete TGFβ, and activate HSCs in turn by an autocrine loop.34) Therefore, TGFβ1 is a promising target for the therapy of liver fibrotic disease.
In a previous study, we demonstrated that PI polyamide targeting TGFβ1 reduced urinary TGFβ excretion by 60%, resulting in marked inhibition of renal injury in an animal model of hypertension-induced renal injury.21) PI polyamide targeting TGFβ1 is designed to inhibit the binding of AP-1 transcription factor to the TGFβ1 promoter. Strong, fast and specific binding of this polyamide to the target sequence was shown in our previous study.19–21) PI polyamide is a novel technology to control specific gene expression alike nucleic acid based medicines. PI polyamide binds to the minor groove of the double helical DNA with high sequence specificity in a hairpin shape forming an amide pairing. PI polyamide recognizes and binds to the target DNA sequence by the recognition rule. The paring of an imidazole opposite a pyrrole targets G-C base pairs, and a pyrrole opposite an imidazole targets C-G base pairs. Pyrrole–pyrrole targets T-A and A-T base pairs.15,16) Following the recognition rules, we can design PI polyamides that bind to target genes playing key roles in diseases for which no effective treatment exists. We have previously reported PI polyamide targeting LOX1,35) ABCA1,23) FcRγ36) and CTGF.37) Whereas nucleic acid medicines such as antisense DNA, ribozymes, and decoys are easily degraded by nucleases, PI polyamides are stable against these enzymes because of their non-nucleic acid based composition and structure. Nucleic acid based medicines require drug-delivery systems for their adequate distribution. By contrast, PI polyamides do not require any transfection regents to incorporate into cells or distribute into target tissues.15,16) Because of these desirable properties of PI polyamide, this novel compound could be used as a gene regulating agent in the near future.
In this study, we did not observe any significant adverse effect due to PI polyamide targeting TGFβ1. However, pharmacokinetics of PI polyamides has not been well examined. Moreover, safety and toxicity of this novel compound has not been fully evaluated. Therefore, further examination of the chemical and biological features of PI polyamides are required in order to develop this compound as a therapeutic agent for human disease.
In conclusion, PI polyamide targeting TGFβ1 suppressed the DMN-induced liver fibrosis. This new compound may be a novel gene regulator for the prevention of liver fibrosis in chronic liver disease.
This work was partially supported by a Grant from the “Strategic Research Base Development” Program for Private Universities subsidized by MEXT (2014).
The authors declare no conflict of interest.