2015 Volume 38 Issue 12 Pages 1918-1924
2015 Volume 38 Issue 12 Pages 1918-1924
We previously reported that dermal application using nanoparticles improves skin penetration. In this study, we prepared novel topical formulations containing ketoprofen (KET) solid nanoparticles (KETnano gel ointment) and investigated the antiinflammatory effect of the KET nanoparticle formulations on rheumatoid arthritis using adjuvant-induced arthritis (AA) rats. The KETnano gel ointment was prepared using a bead mill method and additives including methylcellulose and Carbopol 934; the mean particle size of the KET nanoparticles was 83 nm. In the in vitro skin penetration experiment, the penetration rate (Jc) and penetration coefficient through the skin (Kp) values of the KETnano gel ointment were significantly higher than those of gel ointment containing KET microparticles (KETmicro gel ointment; mean particle size 7.7 µm). On the other hand, in the in vivo percutaneous absorption experiment, the apparent absorption rate constant (ka) and the areas under the KET concentration–time curve values in the skin of rats receiving the KETnano gel ointment were significantly higher than those of rats receiving the KETmicro gel ointment, and the amounts of KET in the skin tissues of rats receiving the KETnano gel ointment were also significantly higher than those of rats receiving the KETmicro gel ointment. In addition, the application of the KETnano gel ointment attenuated the enhancement of paw edema of the hind feet of AA rats more than the application of the KETmicro gel ointment. Our findings suggest that a topical drug delivery system using nanoparticles could lead to expansion in the therapeutic use of KET.
Ketoprofen (KET) is a reversible inhibitor of cyclooxygenases 1 and 2 whose action leads to a reduction in the formation of prostaglandin precursors.1) Thus, KET acts as an anti-inflammatory agent, and is used in the treatment of rheumatoid arthritis (RA) and osteoarthritis. However, its usefulness is limited due to its low water solubility and the fact that oral administration of KET tends to cause gastrointestinal lesions in 10–30% of patients. These gastrointestinal lesions lead to an interruption of drug therapy in about 5–15% patients.2,3) Topical and transdermal delivery routes of drug administration reduce the incidence of gastrointestinal lesions and improve patient compliance. Furthermore, the transdermal route of application avoids hepatic first pass metabolism, meaning that therapeutic serum drug concentrations can be applied with a reduced risk of nephrotoxicity and drug–drug interactions.2) However, the transdermal route of application is limited by the barrier properties of the skin. Therefore, it is necessary to develop enhancement techniques to assist the skin penetration of KET, and extensive research has been done to find both topical and transdermal KET formulations.1,4–6)
To improve the delivery of KET through the skin, several techniques have been employed as follows: liposomes,7) gels,8,9) ointments, creams,10) patches11,12) incorporating various permeation enhancers, micro-needle treatment,13) iontophoresis14,15) and therapeutic frequency sonophoresis (1 MHz).16,17) Recently, strategies using nanoparticles have been developed and investigated as nanomedicines.18–26) The particle size influences their functionality in terms of uptake, residence time in circulation, adherence, and degradation.27–31) Also, we have reported that dermal applications employing nanoparticles enhance skin penetration of the drug.32,33) It is expected that nanoparticles may provide an alternative strategy for improving drug skin permeation,34–37) and it is possible that the therapeutic use of KET may be expanded by the development of a topical drug delivery system using nanoparticles.
Here, we have designed novel topical formulations containing KET solid nanoparticles, and investigated the anti-inflammatory effect of these KET nanoparticle formulations on RA by using adjuvant-induced arthritis (AA) rats. In addition, we demonstrate the enhancement mechanism of skin penetration in drug nanoparticle formulations by comparing the present data with previous reports [skin penetration parameters for gel ointments containing solid tranilast (TL) or indomethacin (IMC) nanoparticles].32,33)
Male 7-week-old Wistar rats, and 6–13-week-old Dark Agouti (DA) rats were used in this study. All animal experiments were carried out in accordance with the Kinki University School of Pharmacy Committee for the Care and Use of Laboratory Animals.
ChemicalsLow-substituted methylcellulose (MC, METOLOSE SM-4, average viscosity, 4 Pa·s at 20°C) was provided by Shin-Etsu Chemical Co., Ltd. (Tokyo, Japan). Carboxypolymethylene (Carbopol® 934) was obtained from Serva (Heidelberg, Germany). Conventional KET (solid, KET microparticles, 7.7±0.28 µm, the mean±standard deviation (S.D.)) and commercially available KET gel (SECTOR gel® 3%) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan) and Hisamitsu Pharmaceutical Co., Inc. (Tokyo, Japan), respectively. All other chemicals used were of the highest purity commercially available.
Preparation of Gel Ointment Containing KET NanoparticlesThe gel ointment containing drug nanoparticles (nano gel ointment) was prepared according to our previous reports.32,33) Conventional KET with MC was crushed with the Bead Smash 12 (a bead mill, Wakanyaku Co., Ltd., Kyoto, Japan),32,33,38–41) to yield milled KET with a particle size of 0.071±0.046 µm (the mean±S.D.). After milling, the KET nanoparticles were added to the Carbopol® 934 gel base (KETnano gel ointment). The Carbopol® 934 gel base was made as follows: Carbopol® 934 (Serva, Heidelberg, Germany) was added to distilled water, allowed to swell for 1 h at room temperature, and neutralized with 5% ammonia water (gel base). The gel ointment containing drug microparticles (micro gel ointment) was prepared by mixing KET microparticles and MC in Carbopol® 934 gel base (KETmicro gel ointment). The formulation of the KET gel ointments is as follows: 3% KET, 0.5% MC, 3% Carbopol® 934, w/w%. The dispersity of the nanoparticles in the ointment base was confirmed as follows: KET gel ointments were divided into 10 parts, and kept at 22°C in the dark for 1 month. The KET particle size in each part was determined by a nanoparticle size analyzer SALD-7100 (Shimadzu Corp., Kyoto, Japan; refractive index 1.70–0.010i). The KET concentration was measured using a Shimadzu LC-20AT system equipped with a column oven CTO-20 A (Shimadzu Corp.) and an Inertsil® ODS-3 (3 µm, column size: 2.1 mm×50 mm) column (GL Science Co., Inc., Tokyo, Japan). The mobile phase consisted of methanol–0.05% trifluoroacetic acid (50 : 50, v/v) at a flow rate of 0.25 mL/min. The column temperature was 35°C, the wavelength for detection was 260 nm, and 1 µg/mL propyl p-hydroxybenzoate was used as an internal standard.
Release of KET from KET Gel Ointments Using a Franz Diffusion CellThe conditions of the drug release experiment from the gel ointments were the same as described in our previous reports.32,33) Membrane filters with pore sizes of 25 and 220 nm (MF™-MEMBRANE FILTER, Merck Millipore, Tokyo, Japan) and a Franz diffusion cell were used in this study. 0.3 g KET gel ointment was spread uniformly over the membrane, which was then mounted in the Franz diffusion cell with 1.6 cm i.d. O-ring flange. The diffusion cells were thermoregulated in a water bath at 37°C for 24 h. One hundred microliter aliquots of sample solution were withdrawn from the reservoir chamber (reservoir volume 12.2 mL), and the KET concentrations in the samples were determined by the HPLC method described above.
In Vitro Skin Penetration of KET Gel OintmentsThe collection of abdominal skin was performed according to our previous reports,32,33) and the conditions of the experiment were the same as the method using the Franz diffusion cell described above. The data obtained were analyzed according to the following equations32,33) (Eqs. 1–3):
 | (1) |
 | (2) |
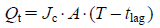 | (3) |
The topical application of 0.3 g KET gel ointment and collection of blood were performed according to our previous reports.32,33) The blood was centrifuged (800×g, 20 min, 4°C), and the KET concentrations in the samples (plasma) were determined by the HPLC method described above. The KET concentration in the plasma after a single injection (650 µg/kg, 0.3 mL) of KET solution containing 1% dimethyl sulfoxide into the femoral vein was calculated according to Eq. 443):
 | (4) |
The percutaneous absorption of KET gel ointment was analyzed according to Eq. 543):
 | (5) |
The area under the KET concentration–time curve (AUC), area under the first moment curve (AUMC) and mean residence time (MRT) were calculated according to the following equations (Eqs. 6–8):
 | (6) |
 | (7) |
 | (8) |
The accumulation of KET gel ointment in the skin tissue was measured according to our previous reports.32,33) The hair on the abdominal area was removed on the day before the experiment, and 0.3 g of KET gel ointment was fixed on the shaved abdominal skin. When collecting the skin tissue, the gel ointment on the skin surface was wiped off, and the abdominal skin to which the KET gel ointments were applied was excised. The skin samples obtained were homogenized in methanol, and the homogenates were centrifuged (20400×g, 20 min, 4°C). The KET concentrations in the supernatants were determined by the HPLC method described above.
Application of KET Gel Ointments to RA Model RatsThe experiment was performed according to our previous reports.32,33) Adjuvant [Bayol F oil containing 10 mg/mL heat-killed Mycobacterium butyricum (Difco, Detroit, MI, U.S.A.)] was injected into the right hind foot and tail of DA rats. The control group received 50 µL of Bayol F oil. The application of KET gel ointments was started after adjuvant injection, and 0.3 g of KET gel ointment was applied to the right foot daily (9:00). When reapplying the gel ointment, the old gel ointment on the skin surface was first wiped off with saline. The inflammation of AA was quantified by the differences in paw volume of the arthritis and normal rats.
The inflammatory scores are represented as AUCedema (the area under the paw volume–time curve) following equation (Eq. 9):
 | (9) |
p Values less than 0.05 were considered significant in this study. Unpaired Student’s or Aspin–Welch’s t-tests were used to determine statistical difference, and multiple groups were evaluated by one-way ANOVA followed by Dunnett’s multiple comparison.
In a previous study, we found that the addition of MC permits the preparation of nanoparticles by mill methods.32,33,38–40) According to these reports, the KET nanoparticles were prepared using MC and Bead Smash 12. Although the bead mill method using KET microparticles produced a meringue state, the addition of MC resulted in the preparation of KET nanoparticles. The prepared KET nanoparticles were of high quality, since the particle size of the milled-KET was less than 100 nm, and the nanoparticles were homogeneous with a narrow particle size distribution (particle size 71±46 nm, mean±S.D.). We also reported that Carbopol 934 allows the uniform incorporation and release of the nanoparticles, and these finding showed that Carbopol 934 is suitable for the preparation of dermal formulations containing nanoparticles.32,33) Therefore, Carbopol 934 was used to prepare a gel ointment containing KET solid nanoparticles. The mean particle size in the 3% KETmicro and KETnano gel ointments was 7.7±0.30 µm, and 0.083±0.071 µm (mean±S.D., Fig. 1), respectively. Carbopol 934 has excellent thickening, emulsifying and gelling properties that allow the uniform incorporation of the KET nanoparticles. The KETmicro and KETnano gel ointments were stable for one month after preparation (mean particle size: KETmicro, 7.8±0.39; KETnano, 0.091±0.083, µm, mean±S.D.), with no decrease in KET concentration in either ointment observed during one month stored at 22°C.

Dashed line, cumulative size distribution; solid line, cumulative size frequency. Mean particle size: KETmicro gel ointment, 7.7±0.30 µm; KETnano gel ointment, 0.083±0.071 µm; mean±S.D.
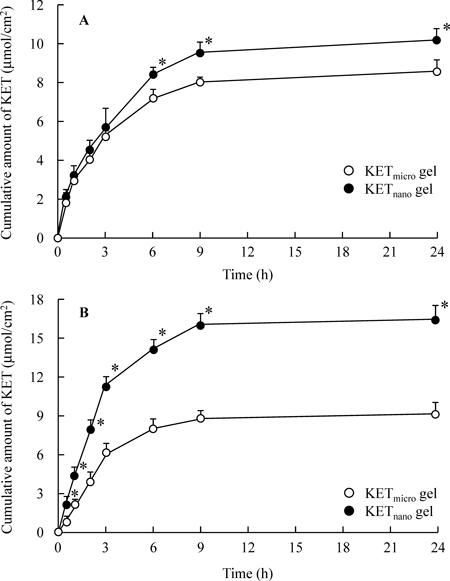
The drug release from and skin penetration of KETnano gel ointments were evaluated. Although KET release from the KETnano gel ointment through the 25 and 220 nm pore size membranes was significantly higher than that from the KETmicro gel ointment, the KET penetration profile of the KETnano gel ointment through the 25 nm pore size membrane was significantly lower than that through the 220 nm pore size membrane (Fig. 2). On the other hand, KET release from the KETmicro gel ointment was similar in experiments using 25 and 220 nm pore size membranes (Fig. 2). This result suggests that the KET released from KETnano gel ointment remains in the nanoparticle state. Figure 3 shows the penetration profiles of KET through rat skin following the application of KETmicro or KETnano gel ointments. The amounts of penetrated KET increased linearly after the application of either KET gel ointment, but the Jc and Kp values of the KETnano gel ointment were significantly higher than those of the KETmicro gel ointment (Table 1). The Km, D and tlag for the KETmicro and KETnano gel ointments showed no significant differences (Table 1). On the other hand, in the in vivo percutaneous absorption experiments using rats, the ka and AUC values in the skin of rats receiving the KETnano gel ointment were significantly higher than those of rats receiving the KETmicro gel ointment (Fig. 4, Table 2). The reduction of tlag and extension of MRT for the KETnano gel ointment also tended to increase in comparison with the KETmicro gel ointment (Table 2). In addition, the amounts of KET in the skin tissues of rats receiving the KETnano gel ointment were significantly higher than those of rats receiving the KETmicro gel ointment (Fig. 5). These results show that the formulation of a topical drug delivery system using KET nanoparticles provides a high level of skin penetration and drug accumulation in skin tissue, and an increase in the percutaneous absorption of KET.
KETmicro gel (open circles): gel ointment containing KET microparticles. KETnano gel (closed circles): gel ointment containing KET nanoparticles. The data represent the mean±S.E. of 6 independent experiments. * p<0.05 vs. KETmicro gel ointment for each category.

KETmicro gel (open circles): gel ointment containing KET microparticles. KETnano gel (closed circles): gel ointment containing KET nanoparticles. The data represent the mean±S.E. of 6 rat skins. * p<0.05 vs. KETmicro gel ointment.
| Ointment | Jc (nmol/cm2/h) | Kp (×10−3 cm/h) | Km | tlag (h) | D (×10−3 cm2/h) |
|---|---|---|---|---|---|
| KETmicro gel | 182±9 | 5.1±0.25 | 0.39±0.13 | 0.88±0.28 | 1.14±0.29 |
| KETnano gel | 316±19* | 8.9±0.55* | 0.61±0.21 | 0.75±0.22 | 1.58±0.43 |
KETmicro gel: gel ointment containing KET microparticles, KETnano gel: gel ointment containing KET nanoparticles. The data represent the mean±S.E. of 6 rat skins. * p<0.05 vs. KETmicro gel ointment for each category.

KETmicro gel (open circles): gel ointment containing KET microparticles. KETnano gel (closed circles): gel ointment containing KET nanoparticles. Solid lines represent the fitted curves for the application of KETmicro and KETnano gel ointments. The data represent the mean±S.E. of 6 rats. * p<0.05 vs. KETmicro gel ointment.
| Ointment | ka (h−1) | tlag (h) | AUC (nmol·h/mL) | MRT (h) |
|---|---|---|---|---|
| KETmicro gel | 0.46±0.16 | 0.43±0.10 | 298±25 | 9.24±1.46 |
| KETnano gel | 1.85±0.42* | 0.27±0.09 | 604±74* | 11.7±1.15 |
α 33.0±5.6 h−1, β 0.47±0.11 h−1, A 10.9±1.87 nmol/mL, B 6.14±0.47 nmol/mL. KETmicro gel: gel ointment containing KET microparticles; KETnano gel: gel ointment containing KET nanoparticles. The data represent the mean±S.E. of 6 rats. * p<0.05 vs. KETmicro gel ointment for each category.

KETmicro gel (open columns): gel ointment containing KET microparticles. KETnano gel (closed columns): gel ointment containing KET nanoparticles. The data represent the mean±S.E. of 6 rats. * p<0.05 vs. KETmicro gel for each category.
It is important to prove the mechanism of the enhanced skin penetration of the topical formulations containing solid nanoparticles. We previously designed gel ointments containing tranilast (TLnano) or indomethacin (IMCnano) nanopariticles (mean particle size: TLnano 79±152 nm, IMCnano 186±101 nm, mean±S.D.), and reported their skin penetration parameters.32,33) The skin penetration profile and drug accumulation in skin tissue were similar for the TLnano and IMCnano gel ointments, and higher than for the micro gel ointments containing TL and IMC (TLmicro and IMCmicro gel ointments).32,33) These results were the same as the current results for the KET gel ointments. In addition, in the in vitro skin penetration experiments, the penetration rate of the IMCnano gel ointment (particle size 186 nm) was higher than that of an ointment containing dissolved IMC (commercially available IMC gel ointment, IDOMETHINEKOWA gel 1%),33) and the skin penetration of the KETnano gel ointment (particle size 83 nm) is also significantly higher than that (Jc 274±13 nmol/cm2/h, n=6) of the ointment containing dissolved KET (commercially available KET gel ointment, SECTOR gel® 3%). Taken together, we hypothesize that drug infiltration into the skin tissue is enhanced for particles in the size range of approximately 80–200 nm in comparison with drugs in the liquid state, and this increase in drug infiltration may cause the high skin penetration and drug accumulation in the skin tissue for the nano gel ointments. In contrast to the results in skin penetration and drug accumulation in skin tissue, the plasma concentration behavior of the TLnano gel ointment differed from that of the IMCnano gel ointment in in vivo percutaneous absorption experiments.32,33) No difference in AUC was observed between the IMCmicro and IMCnano gel ointments. However, the plasma concentration following the administration of the TLnano and KETnano gel ointments was higher than that following the administration of the TLmicro and KETmicro gel ointments. In addition, the plasma concentration following the administration of the TLnano and KETnano gel ointments was lower than that following the administration of the ointments containing the dissolved drugs (commercially available ointments)32); and the AUC of the commercially available KET ointment was 801±51 nmol·h/mL (mean±S.E., n=6 rats). It has been reported that drug solubility can be expected to be enhanced at particle sizes less than 100 nm.44) From these results and reports, it is possible that the solubilities of the drugs in the TLnano and KETnano gel ointments are higher than in the micro gel ointments in skin tissue, while the IMC solubility in the IMCnano gel ointment may not be enhanced since the particle size (186 nm) is over 100 nm. It is hypothesized that solid drugs that infiltrate into the skin tissue are dissolved, and that the liquid drugs can then shift into the blood. On the other hand, it is important to clarify the precise mechanism of the skin penetration of gel ointments containing drug nanoparticles. Therefore, we are now preparing gel ointments containing particles of various sizes, and investigating their characteristics including skin penetration, drug accumulation in skin tissue and percutaneous absorption.
Anti-inflammatory Effects of KETmicro and KETnano Gel Ointments on Paw Edema in AA RatsIn a clinical experiment, a KET gel ointment was used to treat RA. Therefore, we investigated the anti-inflammatory effect of the KETnano gel ointment on RA using AA rats as a model.45–48) Paw edema of the right and left hind feet of AA rats to which the KETmicro and KETnano gel ointments were applied was significantly less in comparison with AA rats treated with gel ointment without KET (control gel ointment) in the days following adjuvant injection. The paw edema of the right and left hind feet of AA rats receiving the KETnano gel ointment was less than that of AA rats receiving the KETmicro gel ointment (Fig. 6). In addition, the AUCedema values of the right hind feet of AA rats treated with the KETnano gel ointment were significantly lower than those of AA rats treated with the KETmicro or control gel ointments (Table 3). These data support the results in which the KET concentrations in the skin tissue and plasma of rats receiving the KETnano gel ointment were higher (Table 2, Figs. 4, 5). Moreover, we measured the anti-inflammatory effect following the application of a commercially available KET gel ointment (SECTOR gel® 3%) to compare it with the KETnano gel ointment. Although the AUCedema values in the right feet of AA rats treated with KETnano gel ointment were significantly lower than those of AA rats treated with the commercially available ointment, the AUCedema values in the left feet of AA rats treated with KETnano gel ointment were higher than those of AA rats treated with the commercially available ointment (right hind feet 33.7±1.5, left hind feet 19.0±1.1 mL·d, the mean±S.E., n=6 rats). From these data, the achievement of relatively high local KET concentrations in the case of the KETnano gel ointment may result in effective therapy, while the systemic influence of the KETnano gel ointment may be lower than that of the commercially available ointment. Further studies are needed in which some mechanistic and/or surrogate biomarkers are carefully monitored.

Vehicle (open circles): gel ointment containing no KET. KETmicro gel (closed circles): gel ointment containing KET microparticles. KETnano gel (closed triangles): gel ointment containing KET nanoparticles. The data are presented as mean±S.E. of 6 independent rats. *1 p<0.05 vs. Vehicle for each category. *2 p<0.05 vs. KETmicro gel ointment for each category.
| Ointment | AUCedema (mL·d) | |
|---|---|---|
| Right | Left | |
| Control | 50.8±2.2 | 32.9±1.5 |
| Vehicle | 50.1±2.5 | 32.5±1.9 |
| KETmicro gel | 41.0±2.2*1,2 | 24.6±1.5*1,2 |
| KETnano gel | 29.3±2.1*1,2,3 | 21.7±1.8*1,2 |
Control: untreated AA rat; Vehicle: AA rat treated with gel ointment without KET; KETmicro gel: AA rat treated with gel ointment containing KET microparticles; KETnano gel: AA rat treated with gel ointment containing KET nanoparticles. The data represent the mean±S.E. of 6 independent rats. *1 p<0.05 vs. Control for each category. *2 p<0.05 vs. Vehicle for each category. *3 p<0.05 vs. KETmicro gel for each category.
In this study, we have developed a novel topical drug delivery system that includes KET nanoparticles. KET released from the KETnano gel ointment accumulates highly in skin tissue, while the plasma KET concentrations are lower than in the case of the commercially available KET gel ointment. In addition, the anti-inflammatory effect on local inflammation of the KETnano gel ointment is significantly greater than those of the KETmicro and commercially available KET gel ointments. The KETnano gel ointment may provide efficient and effective therapy for RA.
The authors declare no conflict of interest.