2015 Volume 38 Issue 3 Pages 389-395
2015 Volume 38 Issue 3 Pages 389-395
The mechanical properties of cells are considered promising biomarkers for the early detection of cancer and the testing of drug efficacy against it. Nevertheless, generalized correlations between drug resistance and the nano-mechanical properties of cancer cells are yet to be defined due to the lack of necessary studies. In this study, we conducted atomic force microscopy (AFM)-based nano-mechanical measurements of cisplatin-sensitive (A2780) and cisplatin-resistant (A2780cis) ovarian cancer cells. The difference in the efficacy of cisplatin between A2780 and A2780cis was confirmed in the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. We observed that the cisplatin-resistant ovarian cancer cells were more motile than cisplatin-sensitive cells based on the results of the wound closure experiment, and the AFM experiments showed that drug resistance induced nano-mechanical stiffening of the ovarian cancer cells. Increased mechanical stiffness caused by cisplatin resistance was consistent with the confocal microscopy images showing more distinct actin stress fibers in A2780cis than in A2780 cells. The down regulation of vinculin implicated the actin-driven elongation as a major motile mode for A2780cis cells. Our results consistently indicated that the acquisition of drug resistance in ovarian cancer cells induces an extensive reorganization of the actin cytoskeleton, which governs the cellular mechanical properties, motility, and possibly intracellular drug transportation.
Ovarian cancer is the leading cause of cancer-related mortality in gynecological malignancies. Little or no specific symptom at the early stage of ovarian cancer hinders the early diagnosis and contributes to the high mortality rate.1) The standard treatment of ovarian cancer is cytoreduction surgery, followed by platinum-based chemotherapy. However, most patients experience re-occurrence of cancer within 2 years of the initial treatment because of cancer cells’ acquisition of resistance against platinum-based chemotherapy. A 5-year survival rate of the advanced ovarian cancer patients is reported to be only about 30%,2) and the acquisition of cisplatin resistance is recognized as a major obstacle against the successful treatment of ovarian cancer. There are still many unresolved questions about how cancer cells become desensitized to chemotherapy.
Recent advances in biomechanical studies suggest that the cellular mechanical compliance plays a crucial role in tumorigenesis and cancer progression.3–5) In recent years, atomic force microscopy (AFM) is finding more attention in the field of biology and pharmaceutics.6–8) Especially, AFM-based nano-mechanics is recognized as a useful tool for detecting mechanical responses from local nanometer-sized domains of a cell.9–11) Changes in mechanical properties during pathological progressions have been investigated using AFM,12,13) and abnormal reorganizations of actin cytoskeletons were noticed for many types of cancer cells.14,15) Not only having an influence on the mechanical compliance, the actin cytoskeleton also affects many cellular processes such as motility, structural stability, cell division, cell–cell adhesion, cell-substrate adhesion, and membrane permeability.16,17) These processes are crucial for cancer cells to migrate through physical and chemical barriers encountered during cancer metastasis.4,18,19) Numerous culprits of cisplatin resistance have been identified, including alterations in apoptotic pathway, induction of drug-detoxifying mechanism, and aberrant regulation of drug transporters that reduces the accumulation of drugs by ejection from the cells.20,21) However, the significance of mechanical alterations on drug resistance is often overlooked. Previous biomechanical studies have shown that the chemotherapeutic agents such as cisplatin, taxol, vincristine, and vinblastine reinforce the mechanical strength of ovarian adenocarcinoma and leukemia cells.22,23) It is also reported that chemotherapy for leukemia patients can cause vascular complications that can lead to lethal conditions.24) Mechanical obstructions in blood vessels partly due to the enhanced cell stiffness and adhesions were noticed as well. Recently, the images taken by stimulated emission depletion (STED) microscopy suggest that the drug resistance of ovarian cancer involves a substantial reorganization of actin filaments.25) Nevertheless, there is a clear lack of understanding on how alterations in mechanical properties and actin organization help cancer cells evade the effects of chemotherapeutic treatments.
In this work, we studied how cellular mechanical properties—mechanical compliance and motility of drug-sensitive ovarian cancer cells differ from drug-resistant ovarian cancer cells using AFM, and the actin cytoskeletal organizations were observed using immunofluorescence microscopy. In order to investigate which component is mainly responsible for the alterations in mechanical properties resulting from the acquisition of drug resistance, nano-mechanical responses of ovarian cancer cells to the treatment of actin-disturbing agents were quantified.
The cisplatin-sensitive (A2780) and the cisplatin-resistant ovarian cancer cells (A2780cis) were obtained from Sigma-Aldrich. Cells were grown in RPMI 1640 with L-glutamine supplemented with streptomycin (500 mg/mL), penicillin (100 units/mL), and 10% fetal bovine serum (FBS) at 37°C and 5% CO2. To retain cisplatin-resistance of A2780cis, 1 µM cisplatin (cis-diamminedichloridoplatinum(II), Sigma-Aldrich) was added to the culture media every 2–3 passages. For AFM experiments, the cells were grown on pre-sterilized glass slides (Erie Scientific, Portsmouth, NH, U.S.A.), 2 d before measurements were taken.
Cell Viability AssayThe comparative sensitivity of A2780 and A2780cis cells to cisplatin was evaluated using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS)-based assay. Cells were seeded at 3000 cells per well in a 96-well plate, the medium volume was set to 100 µL per well, and the cells were allowed to attach overnight. The next day, cisplatin (0, 1, 5, 10, 20, 40, 80, and 100 µM) was added to the wells. Cells were then incubated at 37°C for 72 h. Cell viability was determined using the Cell Titer 96 Aqueous One Solution cell proliferation assay kit (Promega). After incubation with cisplatin, 20 µL of the assay substrate solution was added to the wells, and the plate was incubated at 37°C for an additional 1 h. Absorbance at 490 nm was then measured with Tecan Infinite F200 Pro plate reader, and values were expressed as the absorbance percentage compared to the cells incubated in dimethyl sulfoxide (DMSO) alone.
Phase Contrast MicroscopyWe observed the morphology of cells under an inverted phase contrast microscope (IX-71, Olympus, Japan) with a 20× objective. Images were taken every 24 h in culture for 3 d. We counted the number of cells displaying the stationary and motile shapes. The cells displaying distinct lamellipodia and tails were counted as motile cells. Other cells were counted as stationary. We tracked the change in the cell population of the stationary and motile cells as a function of culture time for each cell line.
Wound Healing AssayFor wound healing assay, cells were seeded in a six-well plate at the density of 1×106 cells/well in the growth medium until they reached 90% confluence. A sterile micro-pipette tip was used to scratch a single wound in each well. The plate was incubated in FBS-free medium with 5% CO2 at 37°C. Images were taken after 0, 6, 12, and 24 h after scratching.
AFM Measurements and Data AnalysisAll AFM measurements were taken with a MFP 3D® (Asylum Research, Santa Barbara, CA, U.S.A.) atomic force microscope equipped with a fluid cell and Bioheater™ operating at 37°C. In order to observe the F-actin disrupting effect on the mechanical compliance, Latrunculin A (LatA) was added to the culture medium 2 h before the data were taken. The data collection was carried out only for the first 3 h after the initial measurement to ensure the healthiness of the cells. V-Shaped silicon nitride cantilevers modified with polystyrene beads were used in order to obtain a well-defined contact area and to reduce the stress from otherwise sharp AFM tips.26) Cantilever spring constants (typically 0.01 N/m) were calibrated by the thermal noise fluctuations method. In order to obtain the elastic moduli, nano-indentation experiments were performed by acquiring f–d curves with a one-second time interval, i.e., 1 Hz, with trigger forces ranging from 150 pN to 300 pN. Measurement points were selected in the region over the center of a cell identified by real time images obtained with the inverted optical microscope (IX-81®, Olympus microscope, Tokyo, Japan) on which the atomic force microscope was installed. As a control experiment, one f–d curve was obtained from a glass slide before taking measurements from the cells in order to make sure of the linear increase in force due the hard glass substrate as a function of z-scanner displacement (d).
In order to determine the elastic moduli, the obtained f–d curves were converted to the force-indentation (f–δ) curves. Force f applied to the cell was calculated by multiplying the spring constant k with the cantilever deflection. The Hertz model was applied to determine the elastic moduli from f–δ curves. According to the Hertz model (Eq. 1), a f–δ curve can be converted to a curve of elastic constant K=E/(1−ν2) versus the dimensionless quantity δ/R, where the R is the radius of the spherical tip and ν is the Poisson ratio. The elastic constant K remains nearly constant as δ/R varied, as expected for a linear homogenous sample.
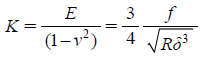 | (1) |
Cells cultured for 72 h on the cover glasses were washed gently with phosphate buffered saline (PBS) and then fixed by immersing the cells in 4% paraformaldehyde for 10 min. The cells were quenched in PBS–NH4Cl for 5 min and then permeabilized with 0.2% Triton X-100 for 5 min. After washing the cells once more, the cells were blocked with 1% bovine serum albumin (BSA) for 10 min. The cells were then incubated at 37°C with 1% phalloidin-fluorescein isothiocyanate (FITC) diluted in PBS-1% BSA for 1 h. After washing the cells four times, the stained cells were mounted in vecta. The images were taken using a confocal microscope (FV1000SPD, Olympus, Tokyo, Japan) with the excitation wavelength of 473 nm using a 100× objective.
Western Blot AnalysisTotal cellular protein extract was isolated from harvested cells, and the protein concentration was determined using Protein Assay Kit (Bio-Rad, Hercules, CA, U.S.A.). Cell lysates (50 µg of total protein) were electrophoresised through 10% denaturing polyacrylamide gels and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA, U.S.A.). The blots were probed with primary antibodies and appropriate secondary antibodies conjugated with HRP (Santa Cruz Biotechnology), and then developed using an enhanced chemiluminescence reagent according to the manufacturer’s protocol. The primary antibodies used were anti-vinculin (Abcam), anti-ILK-1 (Cell Signaling Technology), and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Cell Signaling Technology). The expression level of vinculin and ILK-1 was quantified using ImageJ®.
Statistical SignificanceData were expressed as mean±standard error of the mean (S.E.M.). Statistical significance was identified by Student’s t-test or one-way ANOVA for the difference among treated pairs.
Effect of cisplatin on cell viability was assessed after the exposure of A2780 and A2780cis cells to cisplatin. Cells were treated with varying concentrations of cisplatin (0, 1, 5, 10, 20, 40, 80, and 100 µM) for 72 h. Cell viability of A2780 and A2780cis was measured by MTS colorimetric assay. Cisplatin displayed a dose-dependent cytotoxic effect on A2780 and A2780cis cells. The increase in cytotoxic dose of A2780cis cells confirms the acquisition of cisplatin-resistance (Fig. 1A).

(A) Effect of cisplatin on cell viability. Cells were treated with cisplatin for 72 h. Cell viability was measured by MTS assay. Data are presented as mean±S.D. (n=4). (B, C) Phase contrast images of cells taken after 72 h in culture. While A2780 cells were round and formed colonies (B), A2780cis cells were elongated and triangular (C). The representative shapes of cells were illustrated and labelled as stationary and motile. (D, E) Sub-population of cells according to their morphology. Drug-sensitive ovarian cancer cells were mostly round and stationary (D). However, there was a growing population of motile cells in the drug-resistant cells (E). (F) Images taken from the wound closure experiments. The closures of the straight line-gaps generated at 90% confluent cells were monitored after 0, 6, 12, and 24 h. A significantly more wound closure was observed in A2780cis compared with A7280, indicating the enhanced motile ability of A2780cis cells. * indicates p<0.05.
By phase contrast microscopy, we investigated differences in the morphology between A2780 and A2780cis cells. Motile cells that adhered to the substrate displayed a fan-like shape. They developed distinct lamellipodia consisting of radially oriented actin filaments at the leading edge and tails formed by axial bundles of actin filaments at the rear part of cells. On the contrary, the non-motile and stationary cells lost their directionality and displayed an ellipsoidal or round shape. The phase contrast images showed that A2780 cells were mostly round or ellipsoidal (Figs. 1B–D), but A2780cis cells were mostly fan-shaped (Fig. 1C). A2780 cells showed a stationary shape over the observed time (p<0.05). After 48 h in culture, the population of motile cells was comparable to that of stationary cells for A2780cis (Fig. 1E).
Acquisition of Motile Behavior in Drug-Resistant CellsCell motility is one of the important factors that contribute to metastatic progression. In order to compare the cell motility between A2780 and A2780cis, a conventional wound healing assay was performed.27) A2780 and A2780cis cells were scratched to generate a straight line-gap, and the wound closure was measured after 0, 6, 12, and 24 h after scratching. From four independent experiments, we found that A2780cis cells displayed about 3 times higher migratory behavior compared with A2780 cells. Representative time-lapse images were shown in Fig. 1F. While the wound of A2780 cells stayed unclosed, the wound gap of A2780cis cells was mostly closed after 72 h of the initial scratch. Similar to the result from the cell morphology experiments, the wound closure experiments revealed an enhanced migratory behavior of A2780cis cells compared with A2780 cells.
Nano-mechanical Stiffening Associated with Anti-cancer Drug ResistanceAFM indentation experiments were performed in order to investigate whether the cisplatin-resistance of ovarian cancer cells accompanies biomechanical alterations. We found that force-indentation (f–δ) curves obtained at the centers of A2780 and A2780cis cells agreed well with the Hertz model (Figs. 2A, B). Thus, the elastic constants K could be successfully determined from the Hertz model. Within the observed trigger forces from 150 to 300 pN, A2780 cells underwent significantly larger deformations than A2780cis cells. The maximum indentations of A2780 cells were much bigger than those of A2780cis cells for the same trigger force values, indicating an increased mechanical compliance for A2780 cells.

(A, B) Typical f–d curves obtained at the centers of A2780 and A2780cis cells with the trigger force of 150 pN (A) and 300 pN (B). The solid lines represent the Hertz fitting. Larger indentations, i.e., deformations were observed for A2780 cells for both trigger forces. (C, D) Histogram of Young’s moduli of A2780 (C) and A2780cis cells (D). The Gaussian fits (solid lines) were superimposed. A2780cis cells display a bimodal distribution indicative of heterogeneous mechanical properties.
The average Young’s moduli (mean±S.E.M.) were 80±49 Pa and 273±236 Pa for A2780 (n=37) and A2780cis (n=32) cells, respectively (p<0.001). n represents the number of cells investigated for each cell line for the statistical analysis. In addition to the higher mechanical stiffness, cisplatin-resistant A2780cis cells displayed a bimodal distribution with two main peaks—128±36 Pa and 512±64 Pa (Fig. 2C). However, the drug-sensitive A2780 cells showed a normal Gaussian distribution with one major peak at 82±6 Pa (Fig. 2D).
Mechanical Instability Induced by F-Actin Disrupting AgentsDynamic reorganization of actin cytoskeletons affects cancer progression and the acquisition of drug resistance. In order to investigate which cytoskeletal component is responsible for these functions, we investigated changes in mechanical stability of A2780 and A2780cis cells induced by the F-actin disrupting agent, latrunculin A (LatA).27) The AFM indentation experiments were performed two hours after adding 50 nM of LatA to A2780 and A2780cis cells grown on glass substrates. Typical f–δ curves showed an increased level of indentations for both cell lines after the LatA treatment, indicating the loss of mechanical strength (Figs. 3A, B). Accordingly, we found that the treatment by LatA caused a decrease in the average Young’s moduli to 60±18 Pa (n=34) and 39±20 Pa (n=35) for A2780 and A2780cis cells, respectively. Interestingly, the LatA treatment affected the distribution of Young’s moduli for A2780cis cells. The higher mode in the Young’s moduli distribution curve disappeared after the LatA treatment resulting in both cell lines displaying a normal Gaussian distribution with one nominal peak at the mean value (Figs. 3C, D).

(A, B) Typical f–d curves obtained at the centers of A2780 (A) and A2780cis cells (B) with and without LatA treatment. A significant increase in indentations was noticed for both cell lines. (C, D) Histogram of Young’s moduli of A2780 (C) and A2780cis cells (D) after the LatA treatment. Gaussian fits (solid lines) were superimposed. Young’s moduli of both cell lines showed a normal distribution with one nominal peak.
By performing histochemical staining of actin microfilaments using FITC-phalloidin, we investigated whether the actin arrangement plays a major role in the nano-mechanical alterations caused by cisplatin resistance. The fluorescence images taken by confocal microscopy in Figs. 4A and B showed the actin organization for both cell lines. Both cell lines displayed well-developed actin filaments over the entire cell region. However, prominent actin stress fibers with the polar directional appearance were found in A2780cis cells (Fig. 4A). The actin stress fibers, long and straight bundles of actin filaments, were aligned across the entire region of A2780cis cells. The actin filaments were densely located at the central regions of cells near the nuclei. However, few or no actin stress fibers were present in A2780 cells (Fig. 4B). Short and thin actin filaments were scattered along the peripheral regions of A2780 cells. There was no directional alignment or dense localization of actin filaments in A2780 cells.

(A, B) Fluorescence images of actin microfilaments visualized by confocal fluorescence microscopy after the Phalloidin-FITC treatment. More intensified actin stress fibers were observed in A2780cis cells (A) compared with A2780 cells (B). (C) Representative Western-blot of vinculin and ILK-1, proteins. (D) Quantification of vinculin and ILK-1 expression normalized to GAPDH obtained from the total of six independent experiments. There is about 40% decrease in the expression of vinculin for A2780cis compared with A2780 (* p<0.05) while there is no difference in the expression of ILK-1 between two cell lines.
The Western blot analysis revealed that vinculin was expressed 40% less in A2780cis than A2780 cells (p<0.05) (Figs. 4C, D). Vinculin is a protein in focal adhesions (FAs) involved in cell-matrix adhesion that links the integrin to the actin cytoskeleton.28) However, integrin-linked kinase-1 (ILK-1) was detected comparably for both cell lines. ILK-1 interacts with the cytoplasmic domain of β1-integrin, and is associated with small focal complex which will be matured into larger focal adhesions. Both proteins contribute to the cellular cytoskeletal organization, migration, morphology, and cell survival. The decrease in vinculin by the acquisition of drug resistance is considered to be highly correlated with the actin cytoskeletal reorganization and the changes in motility and morphology.
In order to investigate whether anti-cancer drug resistance is correlated with nano-mechanical compliance, we have determined the Young’s moduli using AFM-based nano-mechanics. Within the observed trigger force of 150 to 300 pN, the drug resistant ovarian cancer cells, A2780cis (273±236 Pa), were mechanically stiffer than the drug sensitive cells, A2780 (80±49 Pa). The mechanical stiffness of both ovarian cancer cells falls in the lower spectrum of the Young’s moduli among cancer cells. According to our previous study, the Young’s moduli of the prostate and breast cancer cells range from 233 to 1055 Pa.29) The mechanical compliance of drug resistant ovarian cancer cells resembles that of the less metastatic prostate cancer cells, LNCaP cells and the drug sensitive ovarian cancer cells are mechanically even more compliant than any other cells measured. Cell migration through the underlying ECM or endothelium is an essential step for cancer metastasis.4) Cells with too high Young’s moduli may have a disadvantage in deforming themselves, but cells with too low Young’s moduli cannot generate enough traction force to penetrate through the surrounding matrix. It is possible that ovarian cancer cells with low mechanical strength belong to the latter case. Our AFM measurements (Fig. 2D) showed that the acquisition of drug resistance is highly correlated with mechanical heterogeneity; A2780cis cells displayed a wider distribution of Young’s moduli than A2780 cells. This result suggests that more A2780cis cells in a given population should be able to generate proper force for invasion and intravasation, possibly resulting in a better chance for escape from the drug treatment.
The changes in nano-mechanical properties induced by the acquisition of drug resistance in ovarian cancer cells agreed well with the actin organization visualized by confocal fluorescence microscopy. We observed more prominent actin stress fibers in the drug resistant A2780cis cells than the drug sensitive A2780 cells (Figs. 4A, B). In addition, our AFM experiments confirmed that a bimodal distribution of Young’s moduli observed in A2780cis cells disappeared after the treatment with an actin disrupting agent (Fig. 3D). These experimental results indicate that drug resistance of ovarian cancer cells induces an extensive reorganization of the actin cytoskeleton involved in the cellular mechanical properties, motility, and possibly intracellular drug transportation.
A rounded bleb-associated motility is promoted by Rho signaling through Rho-associated kinase (ROCK).30,31) On the other hand, elongated cell motility is associated mainly with the protrusions of actin filaments, which does not require Rho and ROCK functions. Vinculin, a molecule associated with focal adhesions, is required to activate the Rho and ROCK signaling and thus the myosin contractility for the bleb-associated cellular migration. The biochemical results from the Western blot analysis showed that vinculin found in focal adhesions was expressed 40% more in the drug sensitive A2780 cells than A2780cis cells (Figs. 4C, D). However, the expression of ILK-1 abundant in small focal complexes was about the same for both cell lines. This biochemical signature supports that the migration of the drug sensitive ovarian cancer cells are directed by the Rho-dependent bleb-associated mode which needs to be activated by vinculin. A recent study on cisplatin-resistant cancer cells showed that Rho GTPase plays a direct role in cell stiffness and drug resistance.32) The increase in the proliferation (data not shown) of A2780 also agrees well with the fact that the contractile motion promotes Erk-based proliferation.33,34) However, our wound healing assay indicates that this bleb-associated migration is not effective for supporting an efficient migration compared to the drug resistant cells likely associated with the elongated actin protrusion. The A2780cis cells driven by the elongated cell motility with the protrusion of actin filaments might be more effective for the motile behavior. Together with the nano-mechanical stiffening optimized for metastasis, the increase in the motility of A2780cis cells could make the cisplatin-treatment for ovarian cancer defeasible by facilitating the invasion and metastasis.
In conclusion, the acquisition of drug resistance is highly associated with significant changes in the actin organization, motile behavior, biochemical signatures associated with adhesion, and mechanical strength. Based on the heterogeneous distribution of mechanical strength resulted from drug resistance, our AFM-based nano-mechanical study can be utilized to help decipher the mechanisms involved in the acquisition of drug resistance.
This research was supported by the Settlement Research Grant of Keimyung University in 2012.