2015 Volume 38 Issue 5 Pages 703-709
2015 Volume 38 Issue 5 Pages 703-709
Luteolin, a flavone found in some vegetables, has been reported to exhibit antioxidant, antiinflammatory, and anticancer activities. In the present study, we found that luteolin has biphasic effects on the viability of the human breast cancer cell line MCF-7. That is, cell viability increased at relatively low luteolin concentrations and decreased at relatively high concentrations. Focusing on the proliferative effect at low concentrations, we showed that luteolin has a cytoprotective effect on MCF-7 cells when administered with doxorubicin. Moreover, luteolin attenuated doxorubicin-induced cytotoxicity even in the presence of the estrogen receptor (ER) antagonist ICI 182,780 and the ER-negative MDA-MB-453 human breast cancer cell line. Reactive oxygen species (ROS) were generated after doxorubicin treatment of MCF-7 cells. In contrast, luteolin attenuated doxorubicin-induced ROS generation. Levels of the antiapoptotic protein Bcl-2 in luteolin-treated MCF-7 cells were significantly higher than those in doxorubicin-treated MCF-7 cells. Our results suggest that a low concentration of luteolin attenuates doxorubicin-induced cytotoxicity to MCF-7 cells through a combination of antioxidant activity and an increase in levels of Bcl-2 protein.
Breast cancer is one of the most common cancers and is the leading type of cancer in Japanese women.1) Over the past several decades, the incidence and mortality rates of breast cancer have been increasing in Japan.2) This increase may be attributed to late marriage and to the declining birthrate.2) The anthracycline antibiotic doxorubicin (adriamycin), one of the most commonly used agents for the treatment of breast cancer, is usually administered with cyclophosphamide and 5-fluorouracil.3–5) Doxorubicin exhibits anticancer activity by intercalation of DNA base pairs and by inhibition of topoisomerase II, which is a key enzyme in the DNA repair system. It has also been shown that doxorubicin can damage DNA through generation of reactive oxygen species (ROS).6) Unfortunately, chronic cardiotoxicity, including development of cardiomyopathies, is a major limiting factor for the chemotherapeutic use of doxorubicin.7) To minimize the side effects of doxorubicin and to improve its chemotherapeutic effects, a wide variety of approaches have been investigated.8–11)
Luteolin (3′,4′,5,7-tetrahydroxyflavone) is a flavone, which is member of the flavonoid group. It is found in its aglycon form in perilla seeds and in its glycosylated form in vegetables such as artichoke, celery, broccoli, cauliflower, green pepper, cabbage, and spinach.12–14) In recent years, there has been increasing interest in the beneficial effects of luteolin. Luteolin has demonstrated antioxidant and anti-inflammatory activities in in vitro and in vivo models.13) The antioxidant activity of luteolin is likely due to its ortho-dihydroxy structure in the B-ring.15) In fact, it has been reported that luteolin scavenged H2O2 in an in vitro assay and inhibited UVB-induced upregulation of cyclooxygenase-2 and prostaglandin E2 in an in vivo model.16) Moreover, luteolin exerts protective effects against cisplatin-mediated nephrotoxicity through regulation of apoptotic pathways in mouse kidneys.17)
In recent years, the anticancer activity of luteolin has been demonstrated in a wide variety of cancer cell lines and in human in vivo models such as colon cancer cells, leukemia cells, hepatoma cells, and hepatocellular carcinoma xenografts.18–20) The proposed molecular mechanisms of the anticancer activity of luteolin involve mediation by cell cycle arrest, activation of apoptosis, and inhibition of tumor invasion.15) There are many reports on anticancer activity of luteolin, but there are also reports on the ability of apigenin (4′,5,7-trihydroxyflavone) at relatively low concentrations to stimulate growth of human MCF-7 breast cancer cells.21) As the structure of luteolin closely resembles that of apigenin, investigators have hypothesized that luteolin also exerts cell-proliferative effects on cancer cells, depending on the experimental conditions. In this study, we showed that luteolin enhances viability of MCF-7 cells and induces cell toxicity, depending on the luteolin concentration in the cells. We then focused on the proliferative effects of luteolin and investigated the effects of luteolin on doxorubicin-induced cytotoxicity to MCF-7 cells.
Details of the cell culture are described in a previous study.22) Briefly, estrogen receptor (ER)-positive MCF-7 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Nihon Pharmaceutical, Tokyo, Japan) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin. The MCF-7 cells were cultured under 100% humidity in a 5% CO2 atmosphere at 37°C. The ER-negative human breast cancer cell line MDA-MB-453 was purchased from Riken BRC Cell Bank (Ibaraki, Japan). MDA-MB-453 cells were cultured in Leibovitz’s L-15 medium (Life Technologies, Carlsbad, CA, U.S.A.) supplemented with 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin. MDA-MB-453 cells were cultured at 37°C in an atmosphere of humidified air without CO2. The medium was changed three times per week.
Cell ViabilityCell viability was assessed by using the Alamar Blue™ assay (Trek Diagnostic Systems, Cleveland, OH, U.S.A.) according to the manufacturer’s instructions. Briefly, cells (10000 cells/100 µL medium) were plated in a 96-well microplate 24 h before use. The culture medium was then replaced with fresh complete medium containing various concentrations of luteolin and doxorubicin for 48 h. Next, Alamar Blue™ solution was added at an amount equal to 10% of the culture volume, and the cells were kept at 37°C in a CO2 incubator for 3 h. The fluorescence of the assay solution was then measured (excitation at 530 nm, emission at 590 nm) with a Fluoroskan Ascent microplate fluorometer (Thermo Scientific, Waltham, MA, U.S.A.).
Mitochondrial MorphologyTo observe the mitochondrial morphology after luteolin and doxorubicin treatment, we used previously constructed MCF-7/pDsRed2-Mito cells generated by transfection of the pDsRed2-Mito® vector (Clontech, Mountain View, CA, U.S.A.) for fluorescent labeling of mitochondria.23) The cells were seeded in two-well Lab-Tek II chamber slides (Nalge Nunc International, Rochester, NY, U.S.A.) 24 h before use. The culture medium was then replaced with fresh medium containing luteolin and doxorubicin for 48 h. The morphology of the mitochondria was observed under an LSM 510 META confocal microscope (Carl Zeiss, Jena, Germany).
Cell CycleTo determine the effects of luteolin on the cell cycle, MCF-7 cells were treated with doxorubicin and luteolin for 48 h. After treatment, the cells were washed with complete medium containing 10% FBS and then washed twice with ice-cold phosphate-buffered saline (PBS)(−). Subsequently, the cells were incubated with 0.1% Triton X-100 in PBS(−) and filtered through a cell strainer. The cells were then incubated with 10 mg/mL RNase and 250 µg/mL propidium iodide for 10 min. The cells were analyzed by using an EPICS-XL (Beckman Coulter, CA, U.S.A.), and data were obtained by using EXPO32 ADC XL 4 Color software (Beckman Coulter).
Detection of ROS in CellsROS production in MCF-7 cells was observed through a fluorescence method based on the oxidation of nonfluorescent carboxy-H2DCFDA (Life Technologies) to green fluorescent dichlorofluorescein (DCF) within the cell. This investigative approach was utilized in another study.24) Cells that had been incubated with luteolin and doxorubicin for 48 h were washed with PBS(−). Carboxy-H2DCFDA (final concentration, 10 µM) was added to the cells, which were then incubated at 37°C for 20 min. The cells were then washed twice with PBS(−) and maintained in complete medium. DCF fluorescence in the cells was monitored under an EVOS fluorescence microscope with a fluorescein isothiocyanate filter (Advanced Microscopy Group, Bothell, WA, U.S.A.).
Western BlotFor the Western blot analysis, MCF-7 cells were lysed with radio-immunoprecipitation assay (RIPA) buffer containing a protease inhibitor cocktail and a phosphatase inhibitor cocktail (Nakalai Tesque, Kyoto, Japan) and then centrifuged at 10000×g for 10 min at 4°C. The supernatants were quantified by using the Pierce BCA protein assay (Thermo Fisher Scientific, MA, U.S.A.). Proteins (10 µg) were separated on 12.5% acrylamide gel and transferred to polyvinylidene difluoride membranes. Blots were probed by using primary antibodies against Bcl-2 (1 : 1000 dilution, Sigma-Aldrich, St. Louis, MO, U.S.A.), p-Akt (1 : 500 dilution, Sigma-Aldrich), Akt (1 : 1000 dilution, Sigma-Aldrich), and β-actin (1 : 1000 dilution, Medical and Biological Laboratories, Aichi, Japan). Subsequently, the membranes were incubated with peroxidase-conjugated anti-rabbit antibody (1 : 2500 dilution, Promega, WI, U.S.A.). Chemiluminescence signals were detected with a VersaDoc 5000 imaging system (Bio-Rad Laboratories, Tokyo, Japan) using the ECL™ Western blotting analysis system (GE Healthcare, Buckinghamshire, U.K.).
RNA InterferenceFor RNA interference, we utilized commercially available short interfering RNA (siRNA) Reagent System (sc-45064, Santa Cruz Biotechnology, MA, U.S.A.) and performed according to the manufacturer’s instructions (Santa Cruz Biotechnology). Briefly, MCF-7 cells were seeded without antibiotics at 300000 cells/well in 6-well plate 18 h prior to transfection. The cells were transfected with 50 nM Bcl-2 siRNA (sc-29214, Santa Cruz Biotechnology) or 50 nM scramble siRNA (sc-37007, Santa Cruz Biotechnology) using siRNA Transfection Reagent (sc-29528, Santa Cruz Biotechnology). Six hours after transfection, add the medium containing luteolin and doxorubicin without removing the transfection mixture and incubate the cells for an additional 48 h. Bcl-2 protein expression was determined by Western blot analysis and cell viability was measured using the Alamar Blue™ assay.
Statistical AnalysisResults are expressed as means±standard deviation (S.D.). The probability of statistical differences between experimental groups was determined by Student’s t-test or by one-way ANOVA followed by Tukey’s honestly significant difference (HSD) post hoc comparisons. KaleidaGraph software was used (Synergy Software, Reading, PA, U.S.A.) to perform Student’s t-test and one-way ANOVA. Statistical differences were considered significant at p values of <0.05.
In order to investigate the effects of luteolin on the viability of MCF-7 cells, the cells were treated with various concentrations of luteolin for 48 h. As shown in Fig. 1A, the viability of MCF-7 cells gradually increased upon treatment with 10 µM luteolin. In contrast, luteolin inhibited cell viability at higher concentrations (above 30 µM). We then assessed the effects of simultaneous treatment with 10 µM luteolin and doxorubicin on the viability of MCF-7 cells. The viability of luteolin-treated cells was significantly higher than that of the control cells (Fig. 1B). Treatment with doxorubicin alone resulted in approximately 50% decrease in cell viability. Simultaneous treatment with luteolin and doxorubicin resulted in increased cell viability compared with that upon treatment with doxorubicin alone. Figure 1C shows representative images of MCF-7 cells after treatment with control, with luteolin, with doxorubicin, and with luteolin and doxorubicin for 48 h. We observed many shrunken cells among the doxorubicin-treated cells. However, shrunken cells among cells cotreated with luteolin and doxorubicin were fewer compared with those among doxorubicin-treated cells. These results suggest that a low concentration of luteolin attenuates doxorubicin-induced cytotoxicity to MCF-7 cells.

(A) Dose-dependent effects of luteolin on the viability of MCF-7 human breast cancer cells. MCF-7 cells were treated with the indicated concentrations of luteolin for 48 h, and the cell viability was then measured by Alamar Blue™ assay, as described in Materials and Methods. (B) Luteolin attenuates doxorubicin-induced cytotoxicity. MCF-7 cells were treated for 48 h with various combinations of 10 µM luteolin and 1.7 µM doxorubicin, and the cell viability was subsequently measured by the Alamar Blue™ assay. (C) Representative images of control cells and of cells after luteolin (10 µM), doxorubicin (1.7 µM), and luteolin–doxorubicin treatments for 48 h. Scale bar=50 µm. Values are expressed as means±S.D. (n=5). * p<0.01 compared with control treatment. # p<0.01 compared with doxorubicin treatment. Cont: control, Dox: doxorubicin, Lut: luteolin.
Previously, we found that treatment with doxorubicin resulted in a characteristic cell morphology such as aggregates of numerous damaged mitochondria on one side of the MCF-7 cells.25) We therefore evaluated the effects of luteolin on the mitochondrial morphology by using previously constructed MCF-7/pDsRed2-Mito cells, which express a red fluorescent protein targeting mitochondria. Figure 2A shows representative fluorescence images of MCF-7/pDsred2-Mito cells cotreated with luteolin and doxorubicin. Mitochondria were distributed uniformly throughout the cytoplasm of control cells. After treatment with doxorubicin for 48 h, mitochondria moderately aggregated on one side of the MCF-7/pDsred2-Mito cells, although the cell membrane was seemingly intact. In contrast, the mitochondrial morphology of cells cotreated with luteolin and doxorubicin was similar to that of the control cells, suggesting that luteolin attenuates mitochondrial damage induced by doxorubicin. As several studies reported that doxorubicin alters the cell cycle distribution in MCF-7 cells, we next investigated the effects of luteolin on the cell cycle by using a flow cytometer.26,27) As shown in Fig. 2B, doxorubicin treatment caused a decrease in the G1 phase (34.9%) as compared with the control (66.4%). In addition, doxorubicin induced cell accumulation in the G2/M phase and slightly increased the sub-G1 phase. On the other hand, cotreatment with luteolin and doxorubicin resulted in a tendency of the G1 phase (39.1%) to increase as compared with that in doxorubicin-treated MCF-7 cells. The increase in G2/M phase induced by doxorubicin tended to decrease compared with that induced by luteolin treatment. Although the cell cycle distribution in cells cotreated with luteolin and doxorubicin shifted slightly toward that of the control cells, cell cycle distributions of cells treated with doxorubicin and of those cotreated with luteolin and doxorubicin were not significantly different. These results suggest that the contribution of the cell cycle to suppression of doxorubicin-induced cytotoxicity is relatively low.
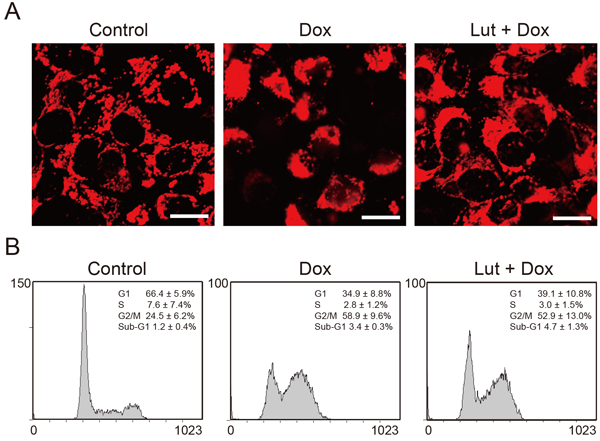
(A) Fluorescence micrographs of MCF-7/pDsRed2-Mito cells after treatment with luteolin and doxorubicin. MCF-7/pDsRed2-Mito cells were treated with 1.7 µM doxorubicin alone or in combination with 10 µM luteolin for 48 h. Scale bar=100 µm. (B) Cell cycles of MCF-7 cells after treatment with luteolin and doxorubicin. MCF-7 cells were treated with 1.7 µM doxorubicin alone or in combination with 10 µM luteolin for 48 h, and the cell cycle was subsequently determined by staining with propidium iodide. Values are expressed as means±S.D. (n=5).
A previous study showed that luteolin possesses potent estrogenic activity; we thus investigated the relationship between estrogenic activity and luteolin levels in MCF-7 cells treated with the ER antagonist ICI 182,780.28) As shown in Fig. 3A, the viability of doxorubicin-treated MCF-7 cells increased with luteolin levels. These results were observed even in the presence of ICI 182,780; there was no statistical significance between cells treated with ICI 182,780 and those without it. We also investigated the viability of ER-negative MDA-MB-453 cells (Fig. 3B). Doxorubicin induced cytotoxicity in a dose-dependent manner. However, cotreatment with luteolin diminished its cytotoxicity. These results suggest that luteolin attenuates doxorubicin-induced cytotoxicity through an ER-independent pathway.

(A) Luteolin attenuates doxorubicin-induced cytotoxicity in the presence of an estrogen receptor (ER) antagonist. MCF-7 cells were treated for 48 h with luteolin and doxorubicin at indicated concentrations with and without 1 µM ICI 182,780. The cell viability was measured by the Alamar Blue™ assay. (B) Luteolin reduces doxorubicin-induced cytotoxicity in ER-negative MDA-MB-453 cells. Cells were treated with doxorubicin at the indicated concentrations alone or with 10 µM luteolin for 48 h. * p<0.01 compared with the same concentration group. Values are expressed as means±S.D. (n=5).
The major pharmacological action of doxorubicin is intercalation with DNA strands, which inhibits the progression of topoisomerase II. However, doxorubicin can damage DNA through ROS generation. As luteolin has antioxidant activity, we hypothesized that luteolin could reduce ROS production induced by doxorubicin treatment of MCF-7 cells. As shown in Fig. 4, increased ROS generation was observed in doxorubicin-treated MCF-7 cells. However, the level of ROS generation in cells cotreated with luteolin and doxorubicin was similar to that of control cells. These results indicate that luteolin attenuates cytotoxicity via reduction of doxorubicin-induced ROS generation in MCF-7 cells.

MCF-7 cells were treated with 1.7 µM doxorubicin alone or in combination with 10 µM luteolin for 48 h, and ROS generation was subsequently observed by using carboxy-H2DCFDA. Scale bar=100 µm.
Recently, investigations demonstrated that luteolin protects against antimycin A-induced cytotoxicity to osteoblastic cells via activation of Akt and against cisplatin-induced nephrotoxicity via upregulation of the anti-apoptotic protein Bcl-2.17,29) Therefore, we believe that luteolin might exert the same effects on doxorubicin-treated MCF-7 cells; we examined Akt and Bcl-2 levels in cells cotreated with luteolin and doxorubicin. As shown in Figs. 5A and B, the total level of Akt was unchanged in the four groups. However, the level of phosphorylated Akt significantly increased in luteolin-treated cells. Bcl-2 levels in luteolin-treated cells were significantly higher than those in control cells, but Bcl-2 levels in doxorubicin-treated and control cells were not significantly different. In contrast, Bcl-2 levels in cells cotreated with luteolin and doxorubicin were significantly higher than those in control cells. To further confirm the effects of Bcl-2 in cells cotreated with luteolin and doxorubicin, we examined the cell viability after Bcl-2 siRNA treatment. In the presence of scramble siRNA, increased Bcl-2 levels were observed in cells cotreated with luteolin and doxorubicin (Fig. 5C). On the other hand, Bcl-2 siRNA treatment caused a decrease in the level of Bcl-2. The cell viability was slightly but significantly decreased in Bcl-2 siRNA transfected cells compared with scramble siRNA transfected cells. These results suggest that luteolin attenuates doxorubicin-induced cytotoxicity via activation of Bcl-2 in MCF-7 cells.

(A) Typical signals of Akt, p-Akt, Bcl-2 and β-Actin were represented in Western blot analysis. MCF-7 cells were treated with combinations of 10 µM luteolin and 1.7 µM doxorubicin for 48 h. (B) Protein expression levels were quantified as described in Materials and Methods. β-Actin was used as a loading control. One hundred percent has been adjusted according to the control group. (C) MCF-7 cells were transfected with scramble siRNA or Bcl-2 siRNA for 6 h followed by treatments of 10 µM luteolin and 1.7 µM doxorubicin for another 48 h. Protein expression levels and cell viability were quantified as described in Materials and Methods. Values from the untreated control were assigned as 100 %. Values are expressed as means±S.D. (n=5). * p<0.01 compared with doxorubicin and luteolin–doxorubicin treatments. ** p<0.05 compared with the control and doxorubicin treatments. ## p<0.01 compared with the scramble siRNA treatment. # p<0.05 compared with the control treatment.
A number of studies have reported that luteolin exhibits activity against a variety of cancer cell lines, such as human colon cancer cells, human leukemia cells, and human hepatoma cells.18–20) We confirmed this anticancer activity in a human breast cancer cell line at relatively high concentrations of luteolin (Fig. 1A). On the other hand, we also found that luteolin stimulated growth of human breast cancer cells at relatively low concentrations (Fig. 1A). Little is known about the proliferative effect of luteolin on cancer cell lines. Therefore, we focused on the proliferative effect of luteolin. We observed suppression of doxorubicin-induced cytotoxicity to MCF-7 cells.
Many dietary flavonoids, including luteolin, are structurally similar to estrogen and possess estrogenic activity that involves a pathway dependent on ERα and ERβ.30) Estrogenic activities of flavonoids are related to their structures, in particular, the number of hydroxyl groups on the B ring of the flavonoid.31) Luteolin has two hydroxyl groups at the 3′ and 4′ positions on the B ring. Therefore, we investigated the relationship between the estrogenic activity and cytoprotective effects of luteolin by using the ER antagonist ICI 182,780 and the ER-negative human breast cancer cell line MDA-MB-453. In contrast to a previous report stating that luteolin exhibits estrogenic activity as a partial agonist, our results show that luteolin has cytoprotective effects on ER-negative cells even in the presence of an ER antagonist.31) The contrasting results may be explained by differences in experimental conditions. Resende et al. reported that the estrogenic activity of luteolin is weak.31) They determined the proliferation of MCF-7/BUS cells, a subline of MCF-7 cells, incubated in luteolin solutions of up to 10 µM for 144 h. Although MCF-7/BUS cells were more sensitive to estrogen than MCF-7 cells, the proliferative effect on luteolin-treated cells was 1.1 times higher than that on control cells. In view of the differences in incubation time and sensitivity to estrogen, we hypothesized that luteolin exerts cytoprotective effects through an ER-independent pathway. As the anticancer activities of doxorubicin are partially due to ROS production, we next investigated whether luteolin exhibits antioxidant activity in doxorubicin-treated MCF-7 cells by using a fluorescence method.6) We observed an increase in ROS generation after doxorubicin administration, which implies that oxidative stress was involved in the death of MCF-7 cells. In contrast to this observation, ROS generation was decreased by the addition of luteolin (Fig. 4). This result indicates that reduction of the pharmacological action of doxorubicin in MCF-7 cells is partially due to the antioxidant activity of luteolin.
We previously reported that administration of doxorubicin in MCF-7 cells resulted in agglomeration of numerous damaged mitochondria on one side of each cell and altered the mitochondrial structure. Such alteration includes conversion of the intact rodlike shape to a round shape, disappearance of cristae and lamellar structures, and appearance of small vesicles inside mitochondria.25) Consistent with our previous report, numerous mitochondria gathered on one side of each doxorubicin-treated MCF-7 cell, as seen in Fig. 2A. We also showed that the mitochondrial morphology of luteolin-treated MCF-7 cells was similar to that of control cells. According to a previous study, luteolin protects osteoblast-like cells from antimycin A-induced cytotoxicity by activating Akt and by improving mitochondrial function.29) Therefore, we hypothesized that luteolin exerts similar effects on doxorubicin-treated MCF-7 cells and investigated the levels of Akt and the anti-apoptotic factor Bcl-2 localized in the mitochondrial membrane. As shown in Figs. 5A and B, phosphorylation levels of Akt and Bcl-2 increased after luteolin treatment. As Akt activation is well known to promote cell survival, our results suggest that the proliferative effect of luteolin at relatively low concentrations might be due to the cell survival properties of Akt. On the other hand, the level of Bcl-2 was significantly increased even in the presence of doxorubicin. Moreover, we observed the decrease of viability after Bcl-2 siRNA treatment in cells cotreated with luteolin and doxorubicin (Fig. 5C). Although Bcl-2 is well known as an anti-apoptotic protein, we previously reported that the features of MCF-7 cell death caused by doxorubicin are not apoptotic.22) One reason for this discrepancy could be attributed to the other functions of Bcl-2. Recent studies have reported that Bcl-2 has multiple functions, including regulation of calcium homeostasis and mitochondrial dynamics, as well as suppression of non-apoptotic cell death, suggesting that various functions of Bcl-2 are involved in the attenuation of doxorubicin-induced cell toxicity of luteolin.32,33) Our findings indicate that luteolin attenuates the pharmacological action of doxorubicin in MCF-7 cells, depending on the experimental conditions.
In conclusion, the aim of the present study was to investigate whether luteolin exerts proliferative effects on the human breast cancer cell line MCF-7. We showed that luteolin exerts both proliferative effects at relatively low concentrations and cytotoxic effects at high concentrations in MCF-7 cells. Our study also showed that a low concentration of luteolin attenuates doxorubicin-induced cytotoxicity to MCF-7 cells. The molecular basis for the cytoprotective effects of luteolin might be reduction of doxorubicin-induced ROS generation and increase in Bcl-2 levels.
This study was supported by the Young Investigator Grant from Ohu University (to Y.S.) and by the Ohu University General Individual Research Grant (to Y.S. and A.U.).
The authors declare no conflict of interest.