2015 Volume 38 Issue 7 Pages 1041-1048
2015 Volume 38 Issue 7 Pages 1041-1048
In this study we describe the design, synthesis, and antibacterial activity of novel pleuromutilin analogs. A series of new compounds containing piperazine and alkylamino or arylamino groups was synthesized. The new compounds were characterized via 1H-NMR, 13C-NMR, Fourier transform (FT)-IR and MS, and were further evaluated for their in vitro activity against seven Gram-positive, and one Gram-negative, pathogens. Antibacterial data revealed that all compounds exhibited moderate to good antibacterial activities against sensitive Gram-positive pathogens. Specifically, 9d displayed the best activity: its activity to Staphylococcus aureus (ATCC25923) is 0.125 µg/mL, which is equal to the control compound tiamulin. The antibacterial activities of 9d to Streptococcus suis (minimum inhibitory concentration (MIC) of 2 µg/mL), Streptococcus agalactiae (MIC of 0.5 µg/mL), and Streptococcus dysgalactiae (MIC of 0.5 µg/mL) were also excellent compared with the control drug erythromycin (MIC of >128 µg/mL). The binding modes of these compounds with active sites were calculated using the programs of Molecular Operating Environment (MOE) and Pymol.
Although countless lives of people have been saved since the discovery of antibiotics, the problem with bacterial resistance to many antibiotics, especially cross resistance, has been increasing. Therefore, we need to identify and develop new antibacterial agents with novel mechanisms of action against bacterial strains.1) Thus far, the use of natural products or semisynthetic derivatives of natural products has been the most successful method of developing new antibiotics.
Pleuromutilin (1) (Fig. 1) was first isolated in 1951 from basidiomycetes Pleurotus and P. passeckerianus, and it exhibits modest in vitro activity against Gram-positive pathogens and mycoplasmas2–4) and weaker in vivo activity. Pleuromutilin has an unusual 5-6-8 tricyclic diterpene structure. This structure was reported by Birch et al. in the 1960 s and was then confirmed via X-ray crystal diffraction technology.5) Further studies have shown that this class of antibiotics interferes with bacterial protein synthesis via a specific interaction with Domain V of the 23S ribosomal RNA (rRNA) of the 50S bacterial ribosome subunit, Cross-resistance with other antimicrobial classes is uncommon.6,7) Specifically, these compounds could bind to the peptidyl transferase center (PTC) of the ribosomal 50S subunit with its tricyclic mutilin core positioned in a tight pocket at the A-tRNA binding site, which subsequently prevents the correct positioning of the CCA ends of tRNAs for peptide transfer.7–9) All of the aforementioned interactions involve prokaryotic ribosomes and not eukaryotic proteins and mammalian ribosomes.4) Based on the structure–activity relationship (SAR) studies, we know that the 5-6-8 tricyclic core is essential for bioactivity, and the carbonyl of C3 and the hydroxyl of C11 are the key groups of antibacterial activity. Moreover it is inactive if a free hydroxyl group at C14 exists.10,11) Several researchers have researched the substituent at C14 in pleuromutilin analogs, and the results revealed that the side chain moiety can maximize the interactions with the peptidyl transferase cavity, and can enhance antimicrobial activity,8,12–14) especially on the basis of the isostere, and replace the O atom at C22 with an S or N atom.
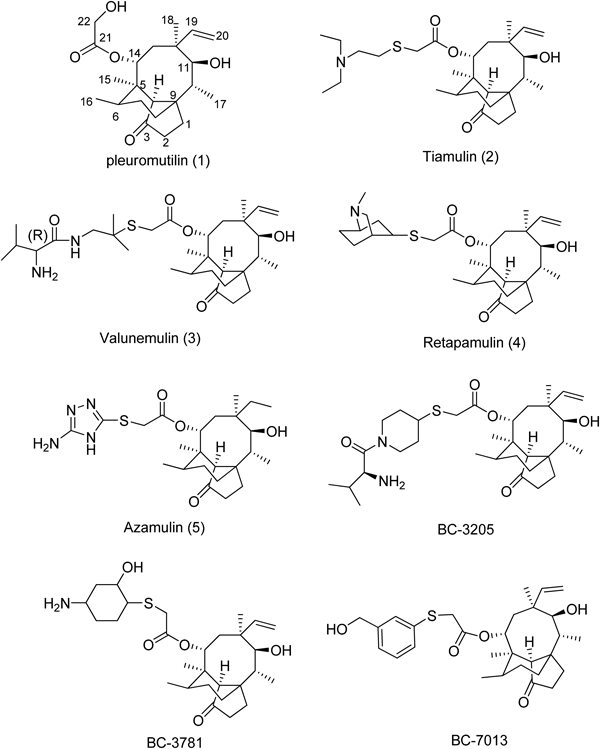
Tiamulin (2) was the first pleuromutilin compound to be approved for veterinary use in 1979. Tiamulin was developed by Sandoz in 1974 as a prophylactic and therapeutic agent for swine dysentery. Valnemulin (3) was launched in 1999 and was used as an effective medicine in the treatment of enzootic pneumonia of pigs. Azamulin (4) was developed in the 1980 s. Although azamulin showed good in vitro activity, its oral bioavailability was severely limited by its poor solubility in water.15) Retapamulin (5) became the first pleuromutilin approved for topical treatment of impetigo, a human skin infection, in April 2007.16) In recent years, several other derivatives have been investigated by medicinal chemists, such as BC-3205 and BC-7013, which are already under Phase I clinical studies, and BC-3781, which became the second pleuromutilin derivative that successfully entered Phase II clinical evaluation.17,18) These results indicate that pleuromutilin derivatives have the potential to be developed as new drugs for human or veterinary use. In recent years, many chemists have devoted themselves to the research on new pleuromutilin derivatives.
Vester and colleagues used the click chemistry, molecular modeling, and chemical footprinting methods to design and synthesize several compounds, which were conjugated with triazole and different nucleoside fragments as side chain.19–21) The importance of lengthening and derivatization of the pleuromutilin side chain has been proven in the molecular level. The team of Hirokawa designed and prepared a series of compounds bearing a purine ring as a polar and water-solubilizing group and a 4-piperidinethio moiety and a piperazine ring as central spacers, and they evaluated the in vitro and in vivo activities of the compounds, several of which exhibited strong in vivo activity.12–14,22) The group of Wang synthesized a series of valnemulin analogs. Several analogs exhibited good in vitro activity.23) The group of Liang designed and synthesized several valnemulin analogs and derivatives, which possessed thiadiazole moieties. These compounds showed moderate to good activity against some Gram-positive pathogens.24,25) Sulfonamides have been used as a C14 side chain by the team of Fang, and these novel hybrid molecules possessed more excellent antibacterial activities than sulfonamides and valnemulin. At the same time, they provide a novel design idea for new pleuromutilin derivatives.26)
We know that the C14 hydroxyl group of pleuromutilin is replaced by a substituent containing the sulfide linkage to show potent in vitro antibacterial activity, but it suffers from rapid in vivo metabolizing because of their strong hydrophobic nature.12) To overcome this problem, the team of Hirokawa designed and synthesized a series of peluromutilin analogs where piperazine was conjugated at C14 as a central spacer, and purine ring as a terminal group to improve water-solubility.
Lipinski et al. established the principle of “the rule of 5” in 1997, which is a set of property criteria that can be used to identify desirable compounds that have good oral absorption. The rule contained four physicochemical properties (H-bond donors <5, H-bond acceptors <10, molecular weight <500, and c log P<5).27) The molecular weight of launched drugs tiamulin, valnimulin and retapamulin is 609, 566 and 516, respectively. The molecular weights of the three investigating compounds are 575, 507 and 500, respectively. Thus, relatively smaller molecular weights maybe beneficial in forming potential drugs. As such, the group of side chain on C14 should not be too large. Inspired by aforementioned information, we designed and synthesized a series of peluromutilin analogs that conjugate piperazine at C14 as a central spacer and alkylamines or arylamines as the hydrophilic terminal group. Compared with the compounds of Hirokawa, our compounds are more suitable for “the rule of 5” (smaller molecular weights and rational H-bond acceptors), and most of the derivatives exhibit good in vitro activity against a number of Gram-positive pathogens.
We designed and synthesized 10 new pleuromutilin derivatives and evaluated their in vitro antibacterial activities against 8 different pathogens based on the National Committee for Clinical Laboratory Standards (NCCLS). All of these compounds exhibited moderate to good antibacterial activities against most Gram-positive pathogens.
All chemicals (analytical grade) used were purchased from Aladdin Reagent and Sinopharm Chemical Reagent Co., Ltd. (China) unless otherwise specified. Separation of the compounds by column chromatography was conducted using silica gel (200 mesh to 300 mesh, Shanghai Shengya Chemical Co., Ltd., Shanghai, China). Petroleum ether and ethyl acetate were used as the mobile phase. IR spectra were obtained using a is10 spectrometer (Nicolet Thermo, Edina, MN, U.S.A.) with KBr thin films. Electrospray ionization (ESI) mass spectra were obtained using the Quattro Micromass MS/MS (Waters Co.). ESI mass spectra were recorded in positive ion mode. 1H-NMR spectra were recorded on a Bruker (Avance 600 MHz; Bruker, Switzerland) spectrometer. The 13C-NMR data were collected on a Bruker instruments (Avance 150 MHz; Bruker, Switzerland) with complete proton decoupling. The chemical shift values (δ) are reported in parts per million (ppm). The internal standard was relative to tetramethylsilane (TMS).
ChemistryThe synthetic routes for all of the derivatives are shown in Chart 1. First, pleuromutilin (1) was reacted with p-toluenesulfonyl chloride (TsCl) in the presence of triethylamine in CH2Cl2 at room temperature for overnight to yield 14-O-(p-toluene sulfonyloxyacetyl) mutilin (6), followed by displacement with piperazine to obtain an excellent yield of 14-O-(1-piperazinyl) mutilin (7) under reflux for 5 h in acetonitrile. The key intermediate 14-O-(1-(4-chloroacetyl piperazinyl)) mutilin (8) was obtained from the reaction of 2-chloroacetyl chloride with 7 in the presence of potassium carbonate, with good yield. Finally, the lead compound 8 was reacted with different substituted alkylamines or arylamines in the presence of potassium iodide (KI) and potassium carbonate (K2CO3) to generate the corresponding 9a–i, with yields of 50 to 70%. The structure of compounds 6, 7, 8, and 9a–i was confirmed via 1H-NMR, 13C-NMR, MS and IR.

Reagents and conditions: (a) p-Toluene sulfonyl chloride, NEt3, DCM, r.t. (b) Piperazine, acetonitrile, r.f. (c) 2-Chloroacetyl chloride, K2CO3, DCM. (d) Alkylamine or arylamine, KI, K2CO3, acetonitrile, r.f.
Pleuromutilin 1 0.7 g (1.85 mmol) and triethylamine 0.28 g (2.8 mmol) were added to 10 mL of CH2Cl2. TsCl 0.53 g (2.8 mmol) was dissolved in 5 mL of CH2Cl2 and dropped into the aforementioned solution. The mixture was then left overnight at room temperature. CH2Cl2 was evaporated in vacuum, and ethyl acetate was then added to the residue. The ethyl acetate extract was washed with brine, and the organic phase was dried 2 h by using anhydrous sodium sulfate, filtered, and evaporated in vacuum. The crude product was recrystallized using ethanol and water. Finally, the pure product 6 was obtained as white solid 0.9 g, with a yield of 91.2%. IR (KBr) cm−1: 3448, 2941, 2864, 1732, 1636, 1597, 1457, 1371, 1224, 1117, 1036, 832, 664, 552. 1H-NMR (CDCl3; TMS) δ: 0.56 (3H, d, J=7.05 Hz), 0.81 (3H, d, J=6.94 Hz), 1.02–1.05 (1H, m), 1.09 (3H, s), 1.19 (1H, d, J=16.10 Hz), 1.26–1.29 (1H, dd, J=2.96, 3.03 Hz), 1.34 (3H, s), 1.37–1.43 (3H, m), 1.52 (3H, s), 1.70 (1H, dd, J=1.98, 1.94 Hz), 1.95–2.01 (2H, m), 2.09–2.23 (3H, m), 2.38 (3H, s), 3.26–3.29 (1H, m), 4.41 (2H, s), 5.14 (1H, d, J=17.4 Hz), 5.27 (1H, d, J=10.99 Hz), 5.71 (1H, d, J=8.57 Hz), 6.32–6.37 (1H, m), 7.29 (2H, d, J=7.91 Hz), 7.75 (2H, d, J=7.65 Hz). 13C-NMR (CDCl3) δ: 11.48, 14.75, 16.53, 21.69, 24.79, 26.35, 26.75, 30.32, 34.39, 35.99, 36.52, 41.81, 43.94, 44.47, 45.37, 58.01, 65.02, 70.25, 74.52, 117.42, 128.09, 129.91, 132.58, 138.66, 145.29, 164.87, 216.75. MS m/z: 555.3 (M+).
14-O-1-Piperazine Mutilin (7)Anhydrous piperazine 0.3 g (3.5 mmol) and K2CO3 0.48 g (3.5 mmol) were added to 10 mL of acetonitrile and stirred under reflux. Compound 6 1.85 g (3.5 mmol) was dissolved in 5 mL of acetonitrile and added dropwise to the aforementioned solution. The mixture was reacted for 5 h, followed by freezing at room temperature. Double volume of water was added and stirred for 1 h. Filtered and the filter cake was washed with water and then placed in a stove at 80°C. Finally, the pure product 7 was obtained as a white solid 1.27 g, with a yield of 82%. IR (KBr) cm−1: 3422, 2930, 2860, 1734, 1458, 1287, 1194, 1153, 1117, 1016, 911, 852. 1H-NMR (d1-CDCl3; TMS) δ: 0.65 (3H, d, J=6.96 Hz), 0.81 (3H, d, J=6.69 Hz), 1.04–1.10 (4H, m), 1.31 (1H, d, J=14.23 Hz), 1.37–1.41 (4H, m), 1.45–1.52 (1H, m), 1.56–1.61 (2H, m), 1.72 (1H, d, J=14.40 Hz), 1.98–2.03 (2H, m), 2.10–2.22 (4H, m), 2.26–2.29 (2H, m), 2.49–2.59 (4H, m), 2.94–2.99 (4H, m), 3.12 (1H, d, J=17.12 Hz), 3.29 (1H, d, J=6.36 Hz), 3.58 (1H, s), 5.15 (1H, d, J=17.39 Hz), 5.23–5.28 (1H, t), 5.73 (1H, d, J=8.43 Hz), 6.42–6.46 (1H, m). 13C-NMR (d1-CDCl3) δ: 11.49, 14.87, 16.71, 24.83, 26.32, 26.80, 30.41, 34.45, 36.01, 36.70, 41.74, 43.92, 44.98, 45.10, 45.44, 52.95, 58.16, 60.17, 68.32, 74.57, 117.30, 139.04, 168.95, 217.11. MS m/z: 447.3 (M+).
14-O-1-(4-Acetylchloride)piperazine Mutilin (8)Compound 7 (1.0 g, 2.24 mmol) and K2CO3 (0.31 g, 2.24 mmol) were added into 10 mL of CH2Cl2 and stirred at room temperature. Chloroacetyl chloride (0.25 g, 2.24 mmol) was added dropwise to the aforementioned mixture and monitored by thin-layer chromatography (TLC). The mixture was concentrated, and the resulting oil was dissolved in 10 mL ethyl acetate. The organic layer was washed with brine, dried using anhydrous Na2SO4, and evaporated in vacuum to obtain white solid 8 (1.10 g), without purification, with a yield of 93.5%. IR (KBr) cm−1: 3462, 2931, 2862, 1734, 1648, 1458, 1193, 1153, 1116, 1011, 913, 792, 660. 1H-NMR (CDCl3; TMS) δ: 0.65 (3H, d, J=7.00 Hz), 0.82 (3H, d, J=6.96 Hz), 1.04–1.09 (4H, m), 1.20 (1H, d, J=10.11 Hz), 1.22–1.23 (1H, m), 1.29–1.32 (1H, m), 1.37 (3H, s), 1.39–1.41 (1H, m), 1.49 (1H, d, J=15.39 Hz), 1.56–1.62 (2H, m), 1.70–1.72 (1H, m), 1.99–2.03 (2H, m), 2.11–2.22 (2H, m), 2.27–2.29 (1H, t), 2.47–2.61 (4H, m), 3.05 (1H, d, J=17.14 Hz), 3.17 (1H, d, J=17.15 Hz), 3.30 (1H, d, J=6.18 Hz), 3.50 (2H, s), 3.61 (2H, s), 3.99 (2H, s), 5.15 (1H, d, J=17.36 Hz), 5.28 (1H, d, J=11.00 Hz), 5.73 (1H, d, J=8.41 Hz), 6.40–6.45 (1H, m). 13C-NMR (CDCl3) δ: 10.51, 13.86, 15.71, 23.83, 25.40, 25.80, 29.40, 33.45, 35.04, 35.67, 40.75, 40.94, 42.93, 44.01, 44.44, 45.08, 51.59, 57.15, 58.51, 67.55, 73.56, 116.29, 138.03, 164.03, 167.80, 216.09. MS m/z: 523.6 (M+).
General Procedure for the Synthesis of Substituted 14-O-1-(4-Acetamido)piperazine Mutilin (9a–i)Compound 8 (1.2 g, 2.30 mmol) and equimolar alkylamines or arylamines were dissolved in 10 mL acetonitrile. K2CO3 (0.19 g, 1.38 mmol) was added as alkali and KI (0.04 g, 0.23 mmol) was added as a catalyzer. The mixture was stirred under reflux for 10 h, followed by evaporation of most of the acetonitrile. Water was added to the residue, and then stirred and filtered. The cake was washed with water, and then placed in a stove at 80°C to obtain the crude product. The crude product was chromatographed on silica gel using petroleum ether and ethyl acetate as the gradient eluent to obtain the pure product.
Mutilin 14-O-1-(4-Dimethylaminoacetyl)piperazine (9a)It was obtained from the mixture of 8, dimethylamine, K2CO3 and KI, with a yield of 65.3%. IR (KBr) cm−1: 3447, 2940, 2825, 1733, 1646, 1457, 1374, 1290, 1194, 1153, 1117, 1011, 731, 668. 1H-NMR (CDCl3; TMS) δ: 0.66 (3H, d, J=7.04 Hz), 0.82 (3H, d, J=7.03 Hz), 1.04–1.10 (4H, m), 1.23 (1H, d, J=15.85 Hz), 1.29–1.32 (1H, m), 1.37 (3H, s), 1.39–1.41 (1H, m), 1.47–1.52 (1H, m), 1.58–1.62 (2H, m), 1.69–1.72 (1H, dd), 1.98–2.03 (2H, m), 2.11–2.22 (2H, m), 2.26–2.29 (7H, m), 2.40–2.55 (5H, m), 3.02 (1H, d, J=17.12 Hz), 3.10–3.14 (3H, m), 3.30 (1H, d, J=6.49 Hz), 3.55–3.60 (4H, m), 5.12–5.15 (1H, dd), 5.26–5.28 (1H, dd), 5.73 (1H, d, J=8.47 Hz), 6.41–6.46 (1H, m). 13C-NMR (CDCl3) δ: 10.49, 13.85, 15.70, 23.82, 25.37, 25.79, 29.39, 33.44, 35.02, 35.67, 40.51, 40.74, 42.92, 43.99, 44.25, 44.43, 51.59, 52.05, 57.14, 58.70, 67.43, 73.54, 116.27, 138.03, 166.86, 167.89, 216.09. MS m/z: 532.3 (M+).
Mutilin 14-O-1-(4-Diethylinacetyl)piperazine (9b)It was obtained from the mixture of 8, diethylamine, K2CO3 and KI, with a yield of 68.3%. IR (KBr) cm−1: 3446, 2934, 2815, 1733, 1635, 1457, 1373, 1290, 1193, 1154, 1117, 1011, 914, 731. 1H-NMR (CDCl3; TMS) δ: 0.66 (3H, d, J=7.04 Hz), 0.82 (3H, d, J=7.01 Hz), 1.00–1.02 (6H, t), 1.06–1.10 (4H, m), 1.23 (1H, d, J=16.01 Hz), 1.29–1.32 (1H, m), 1.37–1.41 (4H, m), 1.47–1.50 (1H, dd), 1.56–1.62 (2H, m), 1.69–1.72 (1H, dd), 1.99–2.03 (2H, m), 2.11–2.22 (2H, m), 2.27–2.29 (1H, t), 2.40–2.53 (4H, m), 2.62 (4H, d, J=6.85 Hz), 3.01 (1H, d, J=17.11 Hz), 3.14 (1H, d, J=17.13 Hz), 3.30 (3H, d, J=6.27 Hz), 3.58–3.61 (4H, m), 5.12–5.15 (1H, dd), 5.26–5.28 (1H, dd), 5.72 (1H, d, J=8.46 Hz), 6.41–6.46 (1H, m). 13C-NMR (CDCl3) δ: 10.47, 13.87, 15.72, 23.83, 25.38, 25.81, 29.41, 33.45, 35.04, 35.69, 40.55, 40.75, 42.94, 43.99, 44.20, 44.45, 46.47, 51.61, 52.02, 57.16, 58.73, 67.46, 73.56, 116.28, 138.05, 167.46, 167.91, 216.11. MS m/z: 560.8 (M+).
Mutilin 14-O-1-(4-Dipropylaminoacetyl)piperazine (9c)It was obtained from the mixture of 8, dipropyl amine, K2CO3 and KI, with a yield of 60.3%. IR (KBr) cm−1: 3412, 2932, 2825, 1738, 1625, 1456, 1373, 1300, 1189, 1151, 1117, 1012, 908, 806, 604. 1H-NMR (CDCl3; TMS) δ: 0.66 (3H, d, J=6.96 Hz), 0.79–0.82 (9H, m), 1.04–1.10 (4H, m), 1.29–1.31 (1H, m), 1.37–1.41 (9H, m), 1.48–1.50 (1H, d, J=14.43 Hz), 1.56–1.62 (3H, m), 1.72 (2H, d, J=14.13 Hz), 1.99–2.03 (2H, m), 2.10–2.22 (2H, m), 2.27–2.30 (1H, m), 2.37 (3H, s), 2.40–2.53 (4H, m), 3.00 (1H, d, J=17.15 Hz), 3.14 (1H, d, J=17.08 Hz), 3.20 (2H, s), 3.29 (1H, s), 3.63 (4H, d, J=29.78 Hz), 5.15 (1H, d, J=17.39 Hz), 5.29 (1H, d, J=10.97 Hz), 5.73 (1H, d, J=8.43 Hz), 6.42–6.46 (1H, m). 13C-NMR (CDCl3) δ: 11.52, 11.91, 14.88, 16.72, 19.88, 24.84, 26.39, 26.81, 30.42, 34.46, 36.04, 36.70, 41.49, 41.75, 43.94, 44.99, 45.19, 45.45, 52.71, 56.23, 58.16, 59.80, 68.42, 74.55, 117.27, 139.04, 168.92, 169.39, 217.07. MS m/z: 588.8 (M+).
Mutilin 14-O-1-(4-Cyclohexylaminoacetyl)piperazine (9d)It was obtained from the mixture of 8, cyclohexylamine, K2CO3 and KI, with a yield of 57.3%. IR (KBr) cm−1: 3435, 2928, 2857, 1732, 1645, 1455, 1373, 1290, 1194, 1153, 1117, 1011, 911, 801, 727. 1H-NMR (CDCl3; TMS) δ: 0.65 (3H, d, J=7.05 Hz), 0.82 (3H, d, J=7.03 Hz), 1.06–1.11 (6H, m), 1.13–1.20 (4H, m), 1.29–1.32 (1H, m), 1.37 (3H, s), 1.39–1.41 (1H, m), 1.47–1.50 (1H, m), 1.52–1.56 (1H, m), 1.57–1.62 (2H, m), 1.66–1.72 (3H, m), 1.80–1.82 (2H, m), 1.99–2.03 (3H, m), 2.11–2.22 (2H, m), 2.27–2.29 (1H, m), 2.35–2.39 (1H, m), 2.43–2.56 (4H, m), 3.02 (1H, d, J=17.15 Hz), 3.15 (1H, d, J=17.16 Hz), 3.29 (1H, d, J=5.44 Hz), 3.37–3.41 (4H, m), 3.60–3.62 (2H, t), 5.12–5.15 (1H, dd), 5.27–5.29 (1H, dd), 5.73 (1H, d, J=8.48 Hz), 6.41–6.46 (1H, m). 13C-NMR (CDCl3) δ: 10.50, 13.86, 15.72, 23.81, 24.92, 25.35, 25.80, 29.41, 32.04, 33.44, 35.04, 35.67, 40.75, 42.93, 43.26, 43.99, 44.44, 46.33, 51.43, 51.67, 56.16, 57.15, 58.65, 67.48, 73.56, 116.33, 138.00, 167.86, 168.07, 216.04. MS m/z: 586.4 (M+).
Mutilin 14-O-1-(4-Pyrrolidinylacetyl)piperazine (9e)It was obtained from the mixture of 8, pyrrolidine, K2CO3 and KI, with a yield of 67.2%. IR (KBr) cm−1: 3420, 2932, 2884, 2809, 1733, 1646, 1457, 1374, 1290, 1192, 1153, 1117, 1011, 914, 732. 1H-NMR (CDCl3; TMS) δ: 0.65 (3H, d, J=7.06 Hz), 0.82 (3H, d, J=7.04 Hz), 1.04–1.10 (4H, m), 1.18–1.23 (2H, t), 1.28–1.32 (1H, m), 1.37 (3H, s), 1.39–1.41 (1H, m), 1.47–1.50 (1H, m), 1.56–1.62 (2H, m), 1.69–1.72 (1H, m), 1.76–1.78 (4H, m), 2.00–2.03 (2H, m), 2.11–2.20 (2H, m), 2.27–2.29 (1H, t), 2.40–2.53 (4H, m), 2.65 (4H, s), 3.02 (1H, d, J=17.13 Hz), 3.14 (1H, d, J=17.14 Hz), 3.30 (1H, d, J=6.45 Hz), 3.36 (2H, s), 3.53–3.54 (2H, t), 3.58 (2H, s), 5.12–5.15 (1H, dd), 5.26–5.28 (1H, dd), 5.73 (1H, d, J=8.46 Hz), 6.41–6.46 (1H, m). 13C-NMR (CDCl3) δ: 10.51, 13.87, 15.72, 22.87, 23.83, 25.38, 25.81, 29.41, 33.45, 35.04, 35.69, 40.51, 40.75, 42.94, 43.99, 44.13, 44.45, 51.56, 56.36, 57.16, 58.70, 67.46, 73.56, 116.29, 138.04, 166.59, 167.90, 216.102. MS m/z: 558.7 (M+).
Mutilin 14-O-1-(4-Piperidylacetyl)piperazine (9f)It was obtained from the mixture of 8, piperidine, K2CO3 and KI, with a yield of 62.9%. IR (KBr) cm−1: 3446, 2934, 2860, 1733, 1635, 1456, 1373, 1304, 1192, 1154, 1118, 1012, 914, 862, 797. 1H-NMR (CDCl3; TMS) δ: 0.73 (3H, d, J=7.05 Hz), 0.89 (3H, d, J=7.02 Hz), 1.13–1.17 (4H, m), 1.30 (1H, d, J=15.92 Hz), 1.36–1.39 (1H, m), 1.42–1.47 (7H, m), 1.55–1.57 (5H, m), 1.63–1.69 (3H, m), 1.76–1.79 (1H, m), 2.06–2.10 (2H, m), 2.18–2.29 (2H, m), 2.34–2.36 (1H, t), 2.44–2.48 (4H, m), 2.51–2.60 (4H, m), 3.06 (1H, d, J=17.08 Hz), 3.14 (1H, s), 3.21 (1H, d, J=17.09 Hz), 3.34–3.37 (1H, m), 3.65–3.68 (4H, m), 5.19–5.22 (1H, dd), 5.34–5.35 (1H, d), 5.80 (1H, d, J=8.48 Hz), 6.49–6.54 (1H, m). 13C-NMR (CDCl3) δ: 11.50, 14.90, 16.72, 23.93, 24.86, 25.95, 26.41, 26.84, 30.45, 34.47, 36.07, 36.72, 41.60, 41.79, 43.97, 45.05, 45.47, 52.77, 53.28, 54.31, 58.19, 59.84, 68.45, 74.58, 117.24, 139.09, 168.39, 168.93, 217.00. MS m/z: 572.4 (M+).
Mutilin 14-O-1-(4-Morpholinylacetyl)piperazine (9g)It was obtained from the mixture of 8, morpholine, K2CO3 and KI, with a yield of 61.7%. IR (KBr) cm−1: 3480, 2929, 2810, 1733, 1634, 1609, 1457, 1290, 1191, 1150, 1117, 1011, 908, 864. 1H-NMR (CDCl3; TMS) δ: 0.66 (3H, d, J=6.97 Hz), 0.82 (3H, d, J=6.89 Hz), 1.04–1.10 (4H, m), 1.23 (1H, d, J=15.99 Hz), 1.29–1.31 (1H, m), 1.37–1.42 (5H, m), 1.45–1.52 (2H, m), 1.72 (2H, d, J=14.18 Hz), 1.99–2.03 (2H, m), 2.10–2.22 (2H, m), 2.25–2.30 (1H, m), 2.43–2.55 (8H, m), 3.02 (1H, d, J=17.14 Hz), 3.10–3.15 (3H, m), 3.28–3.30 (1H, m), 3.56–3.64 (8H, m), 5.15 (1H, d, J=17.40 Hz), 5.29 (1H, d, J=10.97 Hz), 5.73 (1H, d, J=8.43 Hz), 6.41–6.46 (1H, m). 13C-NMR (CDCl3) δ: 11.47, 14.83, 16.68, 24.79, 26.33, 26.77, 30.37, 34.41, 35.99, 36.64, 41.55, 41.71, 43.90, 44.96, 45.33, 45.40, 52.58, 58.11, 59.65, 61.43, 66.82, 68.42, 74.53, 117.26, 138.99, 167.52, 168.85, 217.05. MS m/z: 574.4 (M+).
Mutilin 14-O-1-(4-(N-Methylpiperazinyl)acetyl)piperazine (9h)It was obtained from the mixture of 8, N-methyl piperazine, K2CO3 and KI, with a yield of 65.4%. IR (KBr) cm−1: 3434, 2942, 2880, 2802, 1731, 1627, 1459, 1292, 1191, 1153, 1116, 1012, 983, 915, 830, 727. 1H-NMR (CDCl3; TMS) δ: 0.65 (3H, d, J=6.74 Hz), 0.82 (3H, d, J=7.02 Hz), 1.04–1.09 (4H, m), 1.23 (1H, d, J=16.13 Hz), 1.29–1.31 (1H, m), 1.37–1.41 (4H, m), 1.48–1.50 (1H, m), 1.58–1.61 (2H, m), 1.72 (1H, d, J=14.36 Hz), 1.98–2.03 (4H, m), 2.13–2.21 (5H, m), 2.27–2.28 (2H, d), 2.41–2.52 (9H, m), 3.00 (1H, d, J=17.16 Hz), 3.09–3.14 (3H, m), 3.29 (1H, s), 3.58 (3H, s), 5.15 (1H, d, J=17.38 Hz), 5.28 (1H, d, J=10.86 Hz), 5.72 (1H, d, J=7.79 Hz), 6.41–6.46 (1H, m). 13C-NMR (CDCl3) δ: 10.50, 13.87, 15.73, 23.83, 25.39, 25.81, 29.41, 33.45, 35.03, 35.68, 40.56, 40.75, 42.94, 43.99, 44.35, 44.44, 44.93, 51.96, 52.18, 53.96, 57.16, 58.76, 67.45, 73.56, 116.28, 138.05, 166.91, 167.90, 216.10. MS m/z: 587.4 (M+).
Mutilin 14-O-1-(4-Anilinoacetyl)piperazine (9i)It was obtained from the mixture of 8, aniline, K2CO3 and KI, with a yield of 68.5%. IR (KBr) cm−1: 3396, 2960, 2862, 1734, 1654, 1648, 1604, 1508, 1443, 1420, 1261, 1193, 1152, 1114, 1014, 911, 804, 750, 730, 692. 1H-NMR (CDCl3; TMS) δ: 0.66 (3H, d, J=6.98 Hz), 0.82 (3H, d, J=6.87 Hz), 1.04–1.10 (4H, m), 1.19–1.23 (3H, m), 1.29–1.32 (1H, m), 1.37–1.41 (5H, s), 1.58–1.61 (2H, m), 1.72 (1H, d, J=13.05 Hz), 1.99–2.03 (2H, m), 2.10–2.22 (2H, m), 2.27–2.29 (1H, m), 2.48–2.61 (4H, m), 3.05 (1H, d, J=17.18 Hz), 3.17 (1H, d, J=17.18 Hz), 3.28–3.31 (1H, m), 3.43–3.45 (2H, m), 3.67 (2H, s), 3.80 (2H, s), 5.15 (1H, d, J=17.39 Hz), 5.29 (1H, d, J=10.98 Hz), 5.74 (1H, d, J=8.46 Hz), 6.41–6.46 (1H, m), 6.56 (2H, d, J=7.98 Hz), 6.64–6.67 (1H, t), 7.11–7.13 (2H, t). 13C-NMR (CDCl3) δ: 10.50, 13.85, 15.72, 23.83, 25.36, 25.80, 29.39, 33.43, 35.04, 35.66, 40.74, 40.84, 42.93, 43.99, 44.11, 44.43, 51.54, 57.14, 58.55, 67.55, 73.56, 111.94, 116.33, 116.58, 128.26, 138.00, 146.28, 166.41, 167.76, 216.03. MS m/z: 580.8 (M+).
Staphylococcus aureus (S. aureus) ATCC25923, S. aureus CMCC26003, Staphylococcus epidermidis (S. epidermidis) ATCC12228, Enterococcus faecalis (E. faecalis) ATCC19433, Streptococcus suis (S. suis) ATCC43765, Streptococcus agalactiae (S. agalactiae) ATCC13813, Escherichia coli (E. coli) CVCC231, and methicillin-resistant S. aureus (MRSA) were obtained from Neimeng Province, China.
BioassayThe in vitro antibacterial activities of the compounds were evaluated against a panel of Gram-positive pathogens (S. aureus, MRSA, S. epidermidis, E. faecalis, S. suis, and S. agalactiae) and a Gram-negative pathogen (E. coli) with erythromycin and tiamutilin as reference drugs via the broth micro dilution method based on the National Committee for Clinical Laboratory Standards (NCCLS). The minimum inhibitory concentration (MIC) data are listed in Table 1. The results clearly showed that most of the compounds exhibited moderate to good antibacterial activities. Those activities to S. suis (MIC of 2 to 64 µg/mL) and S. agalactiae (MIC of 0.5 to 8 µg/mL) are excellent compared with that of the control drug erythromycin (MIC of >128 µg/mL). E. coli, as the only Gram-negative pathogen, is insensitive (MIC of >128 µg/mL) to all of the tested compounds, including the control drug. All of the synthesized compounds (MIC of >128 µg/mL) and tiamulin (MIC of 128 µg/mL) are useless against E. faecalis, but erythromycin (MIC of 2 µg/mL) are effective against E. faecalis. Compound 9d showed more potent activities against S. aureus (MIC of 0.125, 0.25 µg/mL), and its activity to MRSA (MIC of 4 µg/mL), S. suis. (MIC of 2 µg/mL), S. agalactiae (MIC of 0.5 µg/mL) and S. epidermidis (MIC of 0.25 µg/mL) are more or less better than those of other compounds.
| Compounds | S. aureus ATCC25923 | S. aureus CMCC26003 | MRSA | S. epidermidis ATCC12228 | S. suis ATCC43765 | S. agalactiae ATCC13813 | E. faecalis ATCC19433 | E. coli CVCC231 |
|---|---|---|---|---|---|---|---|---|
| 9a | 0.25 | 0.5 | 16 | 1 | 8 | 2 | >128 | >128 |
| 9b | 0.25 | 0.5 | 8 | 0.5 | 8 | 2 | >128 | >128 |
| 9c | 0.5 | 0.5 | 8 | 1 | 8 | 2 | >128 | >128 |
| 9d | 0.125 | 0.25 | 4 | 0.25 | 2 | 0.5 | >128 | >128 |
| 9e | 0.25 | 0.5 | 4 | 0.5 | 8 | 4 | >128 | >128 |
| 9f | 1 | 1 | 16 | 1 | 16 | 4 | >128 | >128 |
| 9g | 2 | 2 | 16 | 2 | 32 | 8 | >128 | >128 |
| 9h | 1 | 1 | 32 | 0.5 | 8 | 4 | >128 | >128 |
| 9i | 0.5 | 0.5 | 16 | 1 | 64 | 2 | >128 | >128 |
| 7 | 4 | 8 | 128 | 8 | >128 | >128 | >128 | >128 |
| 8 | 0.5 | 1 | 64 | 1 | 8 | 1 | >128 | >128 |
| Tiamulin | 0.125 | 0.25 | 8 | 0.25 | 2 | 2 | 128 | 64 |
| Erythromycin | 0.06 | 0.125 | 32 | 0.06 | >128 | >128 | 2 | >128 |
a) MIC: The lowest concentration of compound that inhibits visible growth of the organism.
To investigate the relationship of antibacterial activity and the binding mode of the pleuromutilin derivatives with the peptidyl transferase cavity, molecular docking study was performed using software packages MOE 2008.10 and Pymol 1.5.0.3. The docking peptide downloaded from PDB website and the ID number is 1XBP. It is a peptide and ligand (tiamulin) complex, and it applied to build the starting model of the 50S ribosomal subunit.8)
The interaction of compound 9d with the active site of 23S rRNA is presented in pictures A1 and A2 of Fig. 2. There are seven hydrogen bonds between 9d and residues (G2044, C2046, A2430, G2484, and C2565) of the active pocket. Specifically, two hydrogen bonds were formed between the C3 carbonyl and the C11 hydroxyl of mother ring with the N atom of A2430 residue (C=O/NH distance 3.2 Å) and the O atom of G2484 residue (OH/O distance 1.3 Å). The carbonyl group of the C14 side chain formed four hydrogen bonds with the residues of C2046 (C=O/NH distance 3.4 Å), A2430 (C=O/NH distance 3.4 Å), and G2044 (C=O/NH distances 1.9, 2.2 Å). The NH group of cyclohexylamine with the O atom of C2565 formed the last H-bond (NH/O distance 1.7 Å). The picture A2 of Fig. 2 showed that compound 9d docked into the gorge of the 50S ribosomal subunit perfectly, Compound 9f was found to form two hydrogen bonds with the residues of G2484 and A2045 in active pocket (B of Fig. 2). Compared to 9f, compound 9d had an advantage in terms of the number of hydrogen bonds formed with ribosome and had better binding force. Except for compound 9d, other derivatives do not have the hydrogen bond between the C14 side chain and the active pocket residues. The former molecular simulation explained the reason why compound 9d has a better activity than others from a molecular level. The result is consistent with the MIC data. Further, the result preliminary confirmed that the activity of secondary amine is better than that of tertiary amine. Otherwise, compound 9i, which possesses a secondary amine, does not have a similar activity compared with 9d. The result of the molecular docking study of 9i is shown in picture C of Fig. 2, and there are two H-bonds between the O atom of mother ring and the H atom of residues in 23S rRNA through the interaction of tricyclic ring with A2430 (O/NH distance 2.9 Å) and G2484 (O/OH distance 3.4 Å). The side chain of compounds 9d and 9i are both secondary amine, but their binding mode and bioactivities are very different, probably because the electron property is different between the cyclohexyl ring and the benzyl ring, thereby resulting in the conformation is different between them in the pocket space.

The molecular docking results of tiamulin are shown in picture D1 and D2 of Fig. 2. There are three bonds between the O atom of tiamulin and H atom of G2044 (O/NH distance 3.0 Å) and U2544 (O/NH distance 2.9 Å, O/OH distance 3.4 Å) residues. Compound 9d exhibited a docking mode better than that of tiamulin in general, which is inconsistent with MIC data, probably because tiamulin has a good lipid/water partition coefficient in vivo and better binding affinity in dynamic state of 50s ribosomal subunit.28)
In summary, all of the 10 compounds exhibited moderate to good antibacterial activities against most Gram-positive pathogens. The antibacterial activities of these compounds to S. suis (MIC of 2 to 64 µg/mL), S. agalactiae (MIC of 0.5 to 4 µg/mL), and Streptococcus dysgalactiae (S. dysgalactiae) (MIC of 0.5 to 64 µg/mL) are excellent compared with that of the control drug erythromycin (MIC of >128 µg/mL). The activity of compound 9d is the best among all the synthesized analogs, and its MIC to S. aureus (ATCC25923) and S. aureus (CMCC26003) are 0.125 and 0.25 µg/mL, respectively, which is equal to tiamulin. MOE was used in the molecular docking study, and the results showed that all of the new derivatives could bind to the active pocket of the ribosome and that compound 9d could bind better than others. The results are in accordance with the MIC data, thereby indicating the potential of these analogs for further study.
The authors appreciated the help of Key Laboratory of Animal Parasitology of Ministry of Agriculture for providing the laboratory apparatus. This work was supported by the Special Fund for Agro-scientific Research in the Public Interest “Development and application of new type of special animal drugs” (No. 201303038).
The authors declare no conflict of interest.