2015 Volume 38 Issue 7 Pages 1076-1080
2015 Volume 38 Issue 7 Pages 1076-1080
Endoplasmic reticulum stress has been reported to be involved in the pathogenesis of retinitis pigmentosa, macular degeneration and diabetic retinopathy. In the present study, we examined the effects of deferiprone, an iron chelator, on photoreceptor degeneration induced by tunicamycin (300 nmol/eye), an endoplasmic reticulum stress inducer, in the rat retina. Scotopic electroretinogram measurement and morphometric evaluation were done 7 d after the injection of tunicamycin. In the scotopic electroretinogram, intravitreal deferiprone (5 nmol/eye) injected simultaneously with tunicamycin significantly reduced the decreases in a- and b-wave amplitudes induced by tunicamycin. Morphometric evaluation showed that deferiprone significantly reduced thinning of the outer nuclear layer, the inner segment and the outer segment. These results suggest that iron chelation therapy may be a good candidate for the treatment of eye diseases related to endoplasmic reticulum stress.
Retinal degeneration involves various ocular disorders that characterized by the progressive death of retinal neurons. Although the mechanisms of photoreceptor cell death are not fully clarified, endoplasmic reticulum (ER) stress is one of candidates that cause macular degeneration, retinitis pigmentosa and diabetic retinopathy.1) Tunicamycin is an antibiotic that inhibits the biosynthesis of N-acetylglucosaminylpyrophosphoryl polyisoprenol, a key intermediate in the formation of the asparagine-linked oligosaccharides of glycoproteins,2) and widely known to induce ER stress.3) It has been reported that intravitreal tunicamycin causes photoreceptor-specific degeneration.4,5) The intravitreal tunicamycin injection model is used for an animal model of photoreceptor-specific degeneration.6–9)
Increase of reactive oxygen species (ROS) is also known to cause ER stress, Parkinson’s disease,10–12) Alzheimer’s disease,11–13) and photoreceptor degeneration.14) These ROS are believed to be generated at least in part by Fenton reaction, producing hydroxyl radical from hydrogen peroxide. It is well established that labile ferric iron, that does not form a complex with ferritin, is necessary for the Fenton reaction. Previous studies have demonstrated that chemical iron chelators, such as deferoxamine and VK-28, show neuroprotective effects in the animal models of Parkinson’s disease15) and Alzheimer’s disease.16)
The aim of the present study was to determine whether iron chelation protects against photoreceptor degeneration induced by tunicamycin. Deferiprone (DFP), a chemical iron chelator that crosses the blood–brain barrier,17) has shown efficacy with low toxicity in human.18) DFP has also been shown to decrease retinal labile iron without retinal toxicity in the mouse.19) In the present study, we histologically and functionally examined whether intravitreal injection of DFP protects against tunicamycin-induced retinal degeneration.
Experimental procedures conformed to the Regulations for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Kitasato University. Male Sprague-Dawley rats at seven weeks of age were purchased from Japan SLC (Hamamatsu, Japan). The environment of the animal room was kept at 25°C with a 12 h : 12 h light–dark cycle. All animals were fed and watered ad libitum.
Intravitreal InjectionIntravitreal injection was performed as previously described20–23) with some modifications. Briefly, rats were anesthetized with ketamine (90 mg/kg intraperitoneally (i.p.); Daiichi-Sankyo, Tokyo, Japan) and xylazine (10 mg/kg, i.p.; Tokyo Kasei, Tokyo, Japan). Intravitreal injection was performed with a 33-gauge needle connected to a 25-µL microsyringe (MS-N25, Ito Seisakujo, Fuji, Japan). The tip of the needle was inserted approximately 1 mm behind the corneal limbus. Five microliters of the drug solution described below was administered into one eye and the vehicle was administered into another eye as the control.
Preparation of DrugsTunicamycin (Nacalai Tesque, Kyoto, Japan) was dissolved in dimethylsulfoxide. We prepared 2 mg/mL stock solutions of tunicamycin, and diluted them to 120 µg/mL with saline. DFP (Santa Cruz Biotechnology, Dallas, TX, U.S.A.) was dissolved in saline. We prepared 200 µM and 2 mM solutions of DFP. Then, we mixed equal volumes of the two solutions, and 5 µL of the mixed solution was injected intravitreally. Saline containing 3% dimethylsulfoxide or DFP solution containing 3% dimethylsulfoxide was injected to the contralateral eye. The final dose of tunicamycin, which was determined according to previous reports,6–9) was 300 nmol/eye. The final dose of DFP was 0.5 nmol/eye or 5 nmol/eye. According to Berkowitz et al.,24) the volume of the vitreous body in the adult rats is approximately 56 µL. According to Dureau et al.,25) the volume of the vitreous body in a two-month-old rat is approximately 13 µL. Therefore, the calculated intravitreal concentration of DFP is 90–385 µM. In a previous report, 150–300 µM DFP was used to clarify the possible role of iron in the proliferation of osteoblasts.26) The concentration range used in the present study is almost same as that in the previous report.26)
ElectroretinographyScotopic electroretinogram (ERG) was recorded as described previously.27) The rats were dark-adapted overnight. A contact lens electroencephalogram electrode with a white light-emitting diode (Mayo, Inazawa, Japan) was placed in contact with the corneal surface and a reference electrode was placed subcutaneously on the animal’s forehead. A grounding electrode was also placed on the ipsilateral ear. The cornea was intermittently irrigated with a balanced salt solution with Hydroxyethyl cellulose (SCOPISOL®, Senju Seiyaku, Osaka, Japan) to maintain adequate electrical contact and to prevent exposure keratopathy. Long-term pupillary dilation was achieved using topical 1% atropine sulfate (Nihon Tenganyaku, Nagoya, Japan). All electrode placements and eye drop instillations were performed in dim red light so as not to reverse any dark adaptation. Responses to white light flashes (1000 cd/m2, 3 ms) from a photostimulator (LS-W, Mayo) were recorded and measured by PowerLab (AD Instruments, Castle Hill, New South Wales, Australia). The a- and b-wave amplitudes were analyzed using scope software (AD Instruments).
Histological EvaluationHistological evaluation methods have been slightly modified from our previous method.20–23,27,28) Briefly, animals were euthanized with an overdose of sodium pentobarbital. Both eyes were enucleated and fixed with a Davidson solution (37.5% ethanol, 9.3% paraformaldehyde, 12.5% acetic acid) for 24 h at room temperature. Fixed eyes were dissected through the optic nerve head in the vertical meridian with a microtome blade (PATH BLADE+PRO by Kai, Matsunami Glass, Kishiwada, Japan) and embedded in paraffin after the lens had been removed. We used a microtome (HM325, Microm International, Walldorf, Germany) and a microtome blade (PATH BLADE+PRO by Kai, Matsunami Glass) to make 4-µm thickness, horizontal sections through the optic nerve head. Sections were stained with hematoxylin and eosin and examined for morphometry. Using a light microscope (Optiphot-2, Nicon, Tokyo, Japan), the total number of the cells in the retinal ganglion cell layer (GCL) was manually counted in a region beginning 1 mm from the center of the optic nerve head and ending 1.5 mm from the center of the optic nerve head (for a retinal length of 0.5 mm). Digital photographs (digital camera [Senamal, Micronet, Kawaguchi, Japan] connected to a light microscope) were taken so that ca. 0.25 mm of retina appeared in each photograph, with sections ca. 1 mm from the center of the optic nerve head chosen. The thickness of the inner plexiform layer (IPL), the inner nuclear layer (INL), the outer plexiform layer (OPL), the outer nuclear layer (ONL), the inner segment (IS) and the outer segment (OS) was then measured.
Statistical AnalysesAll data are presented as mean±standard error of the mean (S.E.M.). Student’s t-test was used to compare the means of two groups. Tukey–Kramer test was used for multiple comparisons. Differences were considered to be statistically significant if p<0.05.
We investigated the effects of intravitreal DFP on tunicamycin-induced retinal injury in the rat. Typical photomicrographs of the retina taken 7 d after intravitreal injection of tunicamycin are shown in Fig. 1A. Morphometric measurements from 8–12 independent experiments are summarized in Fig. 1B. In the tunicamycin (0.3 µg/eye)-injected control group, the number of the cells in GCL and thickness of IPL did not significantly change. Thickness of OPL and ONL decreased to approximately 60% of those in the contralateral eye. Thickness of IS and OS decreased to approximately 40% of those in the contralateral eye. Intravitreal treatment of DFP (5 nmol/eye) significantly reduced this deleterious effect of tunicamycin. When the lower dose (0.5 nmol/eye) of the drug was administered, such protective effect was not seen. Intravitreal injection of DFP in the absence of intravitreal tunicamycin did not affect the retinal morphology.
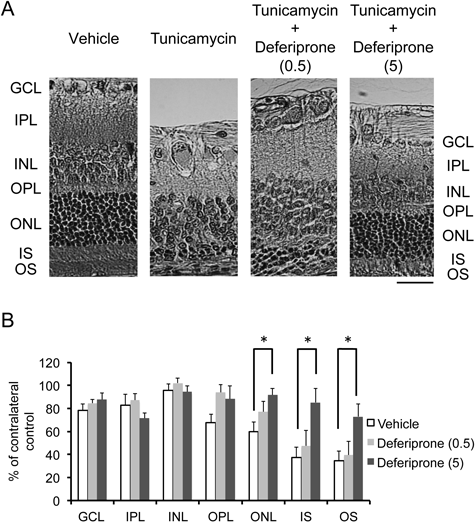
(A) All images were taken 7 d after intravitreal injection. Scale bar=30 µm; Original magnification, ×200. GCL: ganglion cell layer, IPL: inner plexiform layer, INL: inner nuclear layer, OPL: outer plexiform layer, ONL: outer nuclear layer, IS: inner segment, OS: outer segment. (B) The histological Parameters examined were cell density in the ganglion cell layer (GCL) and thickness of the inner plexiform layer (IPL), the inner nuclear layer (INL), the outer plexiform layer (OPL), the outer nuclear layer (ONL), the inner segment (IS), and the outer segment (OS). All measurements in the tunicamycin-injected eyes were normalized to the vehicle-treated contralateral eye and are presented as percentages. Data are presented as the mean±S.E.M. of 12 (vehicle), 10 (0.5 nmol/eye deferiprone) or 10 (5 nmol/eye deferiprone) independent experiments. * p<0.05 vs. the vehicle group. Dunnett’s test was used for multiple comparisons.
Intravitreal injection of DFP in the absence of intravitreal tunicamycin did not affect the amplitudes of scotopic rod-cone a- and b-wave (Fig. 2). The amplitudes of scotopic rod-cone a- and b-wave were vanished by intravitreal tunicamycin. Intravitreal treatment of DFP (5 nmol/eye) significantly reduced the decrease of the amplitudes. The amplitudes were almost half to one third of those in the absence of intravitreal tunicamycin.

(A) Electroretinograms were measured 7 d after intravitreal injection. (B) The data represent the means±S.E.M. of 8 (vehicle), 8 (0.5 nmol/eye deferiprone) or 9 (5 nmol/eye deferiprone) independent experiments. * p<0.05 vs. the vehicle-treated eyes.
In the present study, we have showed that tunicamycin, that is widely known to induce ER stress,3) caused loss of photoreceptor cells (Fig. 1). In addition, we have found that intravitreal injection of tunicamycin induced disappearance of scotopic rod-cone a- and b-wave in electroretinography (Fig. 2). Intravitreal DFP (5 nmol/eye) injected simultaneously with tunicamycin significantly reduced these histological and electrophysiological damages (Figs. 1, 2).
Iron is the most abundant transition metal in the body. It has been reported that the retinal levels of transferrin and ferritin were upregulated in rd10, a mouse model of retinitis pigmentosa.29) Yefimova et al.30) reported that impairment of retinal pigment epithelium–photoreceptor interaction lead to accumulation of iron in the photoreceptor area, and may enhance the vulnerability of cells to oxidative stress in Royal College of Surgeons rat, a rat model of retinitis pigmentosa. In addition, a progressive accumulation of iron and ferritin, the main iron-storage protein, in the substantia nigra has been reported in the Parkinson’s disease patients.31,32) These reports suggest that the increase of iron levels in the neuronal tissues may play an important role in the oxidative-stress-induced neuronal degeneration. Iron is known to be necessary in the conversion of superoxide radical anion, a less-reactive oxygen species, to hydroxyl radical, a highly reactive oxygen radical, by Fenton reaction.33) Such reactive oxygen radicals could attack and injure cell membranes, proteins, and nucleic acids. Therefore, we propose a hypothesis that labile iron could play an important role in development of retinal degeneration.
Our study showed that co-injection of DFP with tunicamycin significantly protected against the tunicamycin-induced loss of neurons in the ONL (Fig. 1), and reduced the decrease of the amplitudes of a- and b-wave of scotopic electroretinogram (Fig. 2). The amplitude of a-wave reflects the physiological function of the photoreceptor cells in the outer retina, and that of b-wave reflects the physiological function of the bipolar cells and the Müller cells, which process the input signals from the photoreceptor cells, in the inner retina. Therefore, DFP partially preserved the function of the photoreceptor cells and/or the bipolar cells and the Müller cells from the tunicamycin-induced injury. The results in the present study demonstrated that retinal degeneration induced by tunicamycin could be partially prevented by iron chelator. DFP has been reported to have good permeability of mitochondrial walls and the blood–brain barrier.17) Although there is no evidence showing that DFP crosses the blood–retinal barrier, it has been reported that transport and permeation characteristics of the blood–retinal barrier are similar to those of the blood–brain barrier.34) It is possible that DFP histologically and functionally protects photoreceptor cells against ER stress-induced injury through chelating labile iron followed by decreased generation of hydroxyl radical. The results in the present study also support the idea that iron-chelation therapy could be one of possible neuroprotective approaches to treat various neurodegenerative diseases related to abnormal iron metabolism in the retina and brain, such as retinitis pigmentosa, Parkinson’s disease and other neurological disorders.35) However, it has been reported that systemic restriction of iron was not neuroprotective in the brain, even though the iron levels in the serum and the brain of the mice fed with the chow without iron were approximately 40% lower than those in the control mice.36) It is possible that mouse has strong compensation mechanism to redistribute iron into the neuron, and/or that the reduction of iron intake was not enough to decrease the cellular iron levels to a critical threshold to stop neuronal degeneration. Further studies are clearly needed to clarify whether systemic restriction of iron is neuroprotective in the retina. Anyway, application of iron chelation may be an effective way to protect retinal rod and cone cells from ER stress-induced degeneration.
In clinical applications, DFP is approved for treatment of thalassemia major in more than 65 countries worldwide, including US, Canada, UK, France and Switzerland. This often requires high doses (75−100 mg per kg body weight orally given in three separate doses per day) continuously administered over extended periods of time. According to “Prescribing Information and Medication Guide” of Ferriprox® (deferiprone) in U.S., the most frequent adverse events reported by patients participating in clinical trials were gastrointestinal symptoms such as nausea, vomiting, and abdominal pain. The discontinuation rate of Ferriprox therapy was reported to be just 1.6% of patients. Ocular toxicity has also been reported in a few cases, including diplopia, papilledema, and retinal toxicity. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or to establish a causal relationship to drug exposure. In the present study, much lower amount of intravitreal DFP (almost 0.7 µg/eye) was effective, and we never observed retinal toxicity in the DFP-injected control eyes. Other researchers reported that DFP decreased retinal labile iron without retinal toxicity in the mouse.19) These results suggest high ocular safety of DFP.
In summary, DFP reduced photoreceptor cell death, leading to both functional and structural rescue in the tunicamycin-induced retinal degeneration model. Unfortunately, it is not expected that DFP can entirely restore photoreceptor cells from degeneration, but we propose that iron chelation therapy may serve for reducing oxidative stress and for enhancing photoreceptor cell survival in retinitis pigmentosa.
This work is supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan, a Grant-in-Aid for Scientific Research (C) #24590329 (KS).
The authors declare no conflict of interest.