2015 年 38 巻 9 号 p. 1328-1336
2015 年 38 巻 9 号 p. 1328-1336
The plant Millettia pulchra was commonly used in folk medicine for the management of inflammation. However, there was no scientific rationale for these effects and the mechanism of action remained incompletely understood. The present study was designed to investigate the antiinflammatory and analgesic activities of an ethanol extract of the stem of M. pulchra (EMP) in vivo, and to explore the antiinflammatory activity of compounds isolated from EMP in vitro. We found that EMP reduced xylene-induced ear edema and relieved both acetic acid-induced pain and pain in the hot plate test. Additionally, a significant decrease in nitric oxide (NO) production was observed in cells treated with the isolated compounds. Lanceolatin B, which showed the greatest inhibition of NO synthesis among the compounds tested, also reduced levels of interleukin-1 beta (IL-1β), IL-6, tumor necrosis factor-alpha (TNF-α), cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), nuclear factor-kappa B (NF-κB), and phosphorylation inhibitory kappa B alpha (p-IκBα) in a dose-dependent manner. These findings provide convincing evidence that EMP and the individual isolated compounds possess significant antiinflammatory and analgesic activities.
Inflammation is a complex biological response of tissues to foreign and noxious stimuli, such as injured cells, mechanical damage, or microbial infection.1) As a protective response, inflammation serves to remove the initial cause of cell injury together with necrotic cells derived from the original insult, and also to initiate the healing process for the recovery of injured tissues. Although inflammation is beneficial for the structural and functional repair of damaged tissue, dysregulation of the inflammatory response may aggravate tissue damage and may also lead to serious chronic diseases, such as pharyngitis, bronchitis and inflammatory bowel diseases.2,3)
The main therapies currently available to treat inflammation and pain are nonsteroidal and steroidal antiinflammatory drugs.4) Nonsteroidal antiinflammatory drugs (NSAIDs), such as aspirin and ibuprofen, are effective for the treatment of a variety of inflammatory and pain conditions5) but their use is limited because of gastrointestinal (GI) toxicity, especially GI bleeding and ulcers.6–8) The use of steroidal drugs has also become highly controversial because of multiple side effects, such as osteoporosis, cataract and obesity.9) Powerful new antiinflammatory agents with fewer side effects would thus clearly benefit those suffering from inflammatory diseases.10) Herbal medicines, which are enormous reservoirs of structurally diverse secondary metabolites,11) are an excellent source of new drugs to treat inflammatory diseases.
The genus Millettia, comprising approximately 200 species, belongs to the Leguminosae family.12) The plant Millettia pulchra KURZ var. LAXIOR (M. pulchra), known as Daluosan in South China, is a plant species commonly used in folk medicine for the management of conditions such as infantile malnutrition, fracture, rheumatism, joint swelling and pain.13) There is, however, no scientific rationale for these effects and the mechanism of action remains incompletely understood. The objective of the present study was to evaluate the antiinflammatory and analgesic activities of an ethanol extract from M. pulchra (EMP) in established animal models and to further explore the mechanism of action using individual compounds isolated from EMP.
The stems of M. pulchra were collected in Guangxi Province, People’s Republic of China, in June 2013, and were taxonomically identified by Professor Lin Yu-Lin. A voucher specimen (No. ALH-13-0618) has been deposited at the Institute of Medicinal Plant Development, Chinese Academy of Medical Science and Peking Union Medical College (People’s Republic of China).
Extraction and IsolationAir-dried stems of M. pulchra (12 kg) were powdered and extracted three times under reflux with 90% aqueous ethanol (EtOH) (3×40 L, 1 h for each extraction). The combined EtOH extracts (120 L) were evaporated under reduced pressure to yield a syrup-like residue (740 g), which was mixed with siliceous earth (80–100 mesh, 800 g) and eluted with hexane. A portion of the evaporated hexane extract (80 g) was subjected to silica gel column chromatography (ϕ7×90 cm) with a gradient elution of hexane–acetone (100 : 1, 50 : 1, 20 : 1, 10 : 1, 5 : 1, 2 : 1, 5 L of each eluent) to give three fractions (A–C). Fraction A (5.2 g) was further purified on a silica gel column eluting with hexane–acetone (ϕ3×50 cm, 50 : 1, 4 L) and then a Sephadex LH-20 column (ϕ1.7×80 cm, CHCl3–MeOH, 1 : 1, 1 L) to afford MPL-06 (10 mg). Fraction B (10 g) was further purified on a silica gel column eluting with hexane–acetone (ϕ4×80 cm, 50 : 1, 6 L), and then a Sephadex LH-20 column (ϕ2×120 cm, CHCl3–MeOH, 1 : 1, 2 L) to obtain MPL-04 (70 mg). Fraction C (20.5 g) was subjected to silica gel column chromatography (ϕ4×80 cm, hexane–acetone, 10 : 1, 6 L), and then purified further on a Sephadex LH-20 column (ϕ2×120 cm, CHCl3–MeOH, 1 : 1, 2 L) to afford MPL-07 (200 mg) and MPL-08 (148 mg). The chromatographic profiles of the components of the EMP were analyzed using high performance liquid chromatography (HPLC) as shown in Fig. 1(A). HPLC analyses were performed on a Waters 600 liquid chromatographic system equipped with a Waters 600 pump and a Waters 2487 dual l absorbance detector. The HPLC system consisted of a Merck Purospher Star C-18 column (4.6–250 mm i.d., 5 mm particle size) with a mobile phase consisting of acetonitrile–water (55 : 45). The flow-rate was 1-mL/min and UV detection was carried out at 271 nm. Based on analyses of the UV, IR, NMR, and MS spectra, together with measurements of physicochemical parameters, chemical structures were assigned as follows: ovalitenin B (MPL-04); ovalitenin A (MPL-06); lanceolatin B (MPL-07); 2″,2″-dimethylpyrano-[5″,6″:7,8]-flavone (MPL-08) as shown in Fig. 1(B).
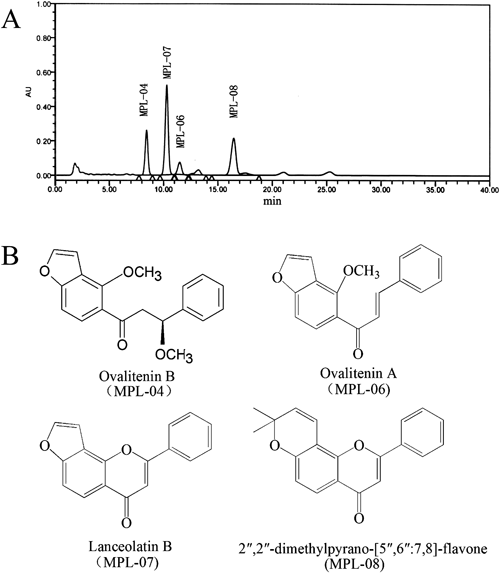
Each peak in the HPLC profile was identified by comparison of the retention times and UV spectra of chemically defined standard compounds. The peaks indicate the following: ovalitenin B (MPL-04); lanceolatin B (MPL-07); ovalitenin A (MPL-06); 2″,2″-dimethylpyrano-[5″,6″:7,8]-flavone (MPL-08).
ICR male and female mice weighing 18–22 g were purchased from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Animals were housed in a regulated environment (25±2°C), with a 12 h light/dark cycle and received water and food ad libitum. The animal protocol was approved on April 15, 2015 (No. 15-04-15) by the Animal Ethics Committee at the Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences.
To investigate antiinflammatory and analgesic activities of EMP in vivo, mice were divided into five groups of six mice each. The test groups were orally pretreated with EMP (25, 50 and 100 mg/kg), aspirin (100 mg/kg) and saline solution (control) for 7 consecutive days.
Cell Lines and ReagentsRAW264.7 cell lines were obtained from Chinese Academy of Medical Sciences Basic Medicine Cell Center (Beijing, China). Primary antibodies of nuclear factor-kappa B (NF-κB), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), phosphorylation-inhibitory kappa B alpha (p-IκBα) and β-actin, as well as all secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Mouse interleukin-6 (IL-6), IL-1β and tumor necrosis factor-alpha (TNF-α) enzyme-linked immunosorbent assay (ELISA) Kits were products of Abcam, Inc. (Cambridge, U.K.). Griess Reagent was obtained from Enzo Life Sciences, Inc. (Beijing, China). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco-BRL (Grand Island, NY, U.S.A.). 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), indomethacin and lipopolysaccharide (LPS) (Escherichia coli, serotype 0111:B4) were purchased from Sigma Co., Ltd. (St. Louis, MO, U.S.A.). Aspirin was purchased from Bayer Pharma AG (Leverkusen, Germany). All other chemicals and reagents used in this study were of analytical grade.
Cell CultureRAW264.7 macrophages were maintained in DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37°C in an atmosphere of 5% CO2. For all experiments in this study, the cells were grown to 80–90% confluence and they were subjected to no more than 20 cell passages.
Antiinflammatory and Analgesic Activities in VivoXylene-Induced Ear Edema in MiceThe xylene-induced ear edema test was conducted according to the method described previously,14) with minor modification. Thirty minutes after the final administration of drugs, each animal was received 20 µL xylene (Beijing Chemical Works, Beijing, China) to the inner and outer surface of the right ear, while the left ear kept untreated. One hour later, mice were sacrificed, and a stainless steel punch with diameter of 6 mm was used to take the central sections of the right and left ears. The sections of both the right and left ears were weighed, and the weight difference was used to evaluate ear edema. The ear edema percent was calculated using the following formula:
 |
The acetic acid-induced writhing test15) was conducted in mice to evaluate the peripheral analgesic activity of EMP. Thirty minutes after the final administration of drugs, mice were intraperitoneally injected with 0.6% acetic acid (v/v, 10 mL/kg). Then the mice were placed in separated boxes, and the total number of writhes occurring between 0 and 30 min after the injection of acetic was counted to quantify the pain intensity. The time that elapsed until the occurrence of writhing was recorded as the latency of pain response.
Hot Plate TestThe hot plate test was conducted as described elsewhere.16,17) In this test, mice were placed on the hot-plate maintained at 55±0.5°C. The time that elapsed until the occurrence of either a hind paw licking or a jump off the surface was recorded as the latency of pain response. The cut-off time was set at 60 s to avoid damage to the paw. Mice with latencies of below 5 s or over 60 s were removed from the study. After the determination of pain response latency, measurements were conducted at 30, 90 and 150 min after the final dose of drugs, respectively.
Antiinflammatory Activities in VitroCell Viability AssayRAW264.7 macrophages were plated in a 96-well plate at a density of 1×104 cells/well and incubated at 37°C for 24 h. RAW264.7 macrophages were then treated with ovalitenin B, ovalitenin A, lanceolatin B and 2″,2″-dimethylpyrano-[5″,6″:7,8]-flavone at various concentrations, and the cell viability was measured by using MTT method.18,19) After indicated treatments for 24 h, an MTT solution was added to each well at the final concentration of 200 µg/mL, and the cells were incubated for another 4 h at 37°C in an atmosphere of 5% CO2. At the end of the incubation, the supernatant was removed and 100 µL dimethyl sulfoxide (DMSO) (Sigma-Aldrich) was added to solubilize the formazan. The absorbance was measured at 570 nm by using a microplate reader. The untreated cells were considered as 100% viable cells. Results are expressed as percentage of viable cells when compared with the control group.
NO AnalysisNO was determined by using Griess reagent.20) RAW264.7 cells were plated at a density of 1×105 cells/well in a 96-well plate with 100 µL of culture medium and incubated for 4 h, and then treated with LPS (1 µg/mL) and various concentrations of ovalitenin B, ovalitenin A, lanceolatin B and 2″,2″-dimethylpyrano-[5″,6″:7,8]-flavone for 24 h. A hundred microliters of the cell culture supernatant was mixed with 100 µL of Griess reagent (mixture of equal amounts of reagents A and B, A: 1% sulphanilamide in 5% H3PO4, B: 0.1% naphthylethylene diamine dihydrochloride), followed by incubation for 10 min at room temperature. The absorbance was measured at 540 nm by using a microplate reader, and a standard calibration curve prepared from different concentrations of sodium nitrite was used to calculate the inhibitory rates.
Measurement of Proinflammatory Cytokine (IL-1β, IL-6, TNF-α)RAW264.7 macrophages (1×105 cells/well) were cultured for 4 h in flat-bottom 96-well plates after which LPS (1 µg/mL) with or without lanceolatin B was added to the cultured cells and incubated for a further 24 h. At the end of the incubation, IL-1β, IL-6 and TNF-α levels in the medium were measured using a mouse ELISA kit (Abcam, Cambridge, U.K.) specific for each cytokine. All experiments were performed in triplicate.
Assay of COX-2, iNOS, NF-κB and p-IκBαWestern blot21) was used to determine the expression of COX-2, iNOS, NF-κB and p-IκBα in LPS-stimulated RAW264.7 macrophages. RAW264.7 macrophages were plated at a density of 1×106 cells/well in a 6-well plate with 2 mL of culture medium, and incubated at 37°C for 24 h. The macrophages were then treated with LPS (1 µg/mL) with or without lanceolatin B. At the end of the treatment, cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed in a cold lysis buffer (10% NP-40, 10 mM Tris, 150 mM NaCl, 2 mM phenylmethylsulfonyl fluoride (PMSF), 5 µM leupeptin, pH 7.6). The cell lysates were centrifuged at 12000×g at 4°C for 10 min to obtain total protein in supernatant. Protein concentrations were determined by the method of bicinchoninic acid (BCA) using a commercial kit (Cowin Biotech Co., Ltd., Beijing, China). Aliquots of the lysates (30–40 µg of protein) were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride membrane. After blocking with 5% nonfat dried milk in Tris buffered saline-Tween (TBST, 20 mM Tris–HCl, 150 mM NaCl, 0.05% Tween 20), the membrane was then incubated with the first specific antibody (Santa Cruz Biotechnology) in blocking solution (5% nonfat dried milk, 1 : 1000) at 4°C overnight. The membrane was washed with TBST and further incubated for 1 h with horseradish peroxidase-conjugated secondary antibody solution (Santa Cruz Biotechnology, 1: 3000) at room temperature. The protein bands were washed thrice with TBST and detected using an enhanced chemiluminescence ECL (Cowin Biotech Co., Ltd.). All experiments were performed in triplicate.
Statistical AnalysisAll results in this study were expressed as the mean±standard error of the mean (S.E.M). The data obtained were statistically analyzed using one-way ANOVA, followed by Dunnett’s multiple comparison tests. Differences were considered significant when p≤0.05.
To evaluate whether EMP possesses antiinflammatory activity, a xylene-induced mouse ear edema model was used. It was observed that EMP (25, 50, 100 mg/kg) showed significant (p<0.01) inhibition of the xylene-induced mice ear edema in a dose-dependent manner as compared to those results obtained from control groups (Fig. 2), with ear edema percents ranging from 52.9 to 31.1%. The ear edema percent of aspirin (100 mg/kg) was 50.1% (Fig. 2).

Animals were pretreated by oral administration of EMP (25, 50 and 100 mg/kg), aspirin (100 mg/kg) or vehicle for 7 consecutive days. Data are expressed as mean±S.E.M. N=6 mice per group. ** p<0.01 compared with vehicle control.
The analgesic activity of EMP was initially evaluated using the writhing test. Oral administration of EMP (25, 50, 100 mg/kg), 30 min before the acid injection, produced a significant (p<0.01) and dose-dependent inhibition of acetic acid-induced abdominal constrictions in mice (Fig. 3A). Aspirin (100 mg/kg), a standard NSAID used as positive control, also produced significant inhibition (p<0.01) of acetic acid-induced writhing response. The latency time also increased significantly (p<0.01) in the EMP group (50, 100 mg/kg), and the latency time (737 s) with treatment of EMP 100 mg/kg was almost twice as long as that in the vehicle control group (427 s) (Fig. 3B). The analgesic activity of EMP (25, 50, 100 mg/kg) in the hot plate test was shown in Fig. 4. When mice were treated with EMP (100 mg/kg), a significant (p<0.05) analgesic activity was observed 30 min and 90 min after the final dose compared with the vehicle control group. However, aspirin at 100 mg/kg did not offer any protection against the heat induced pain (Fig. 4).
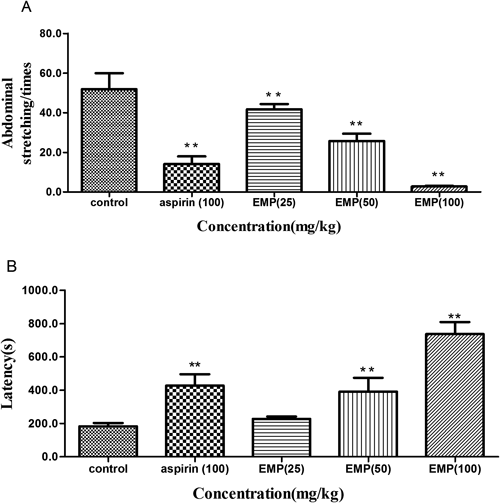
Animals were pretreated by oral administration of EMP (25, 50 and 100 mg/kg), aspirin (100 mg/kg) or vehicle for 7 consecutive days. Abdominal stretching times (A) and latency (B) were recorded in this measurement. Data are expressed as mean±S.E.M. N=6 mice per group. ** p<0.01 compared with vehicle control.
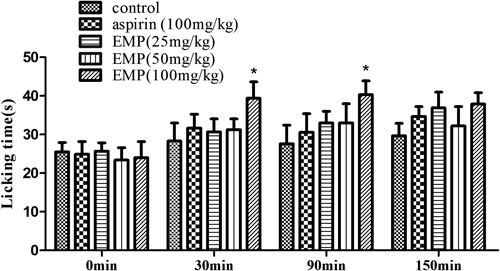
Animals were pretreated by oral administration of EMP (25, 50 and 100 mg/kg), aspirin (100 mg/kg) or vehicle for 7 consecutive days. Measurements were performed beforehand and at 30, 90 and 150 min after the final dose of drugs, respectively. Data are expressed as mean±S.E.M. N=6 mice per group. * p<0.05 compared with vehicle control.
The cytotoxic effects of compounds isolated from EMP are shown in Fig. 5. Concentrations that caused no more than 20% loss of cell viability were determined to be 80, 45, 380 and 40 µM for ovalitenin B, ovalitenin A, lanceolatin B and 2″,2″-dimethylpyrano-[5″,6″:7,8]-flavone, respectively (Fig. 5). Studies to analyze the antiinflammatory activities of individual compounds were carried out at concentrations lower than these since we speculated that such small losses of cell viability would not affect further investigations.
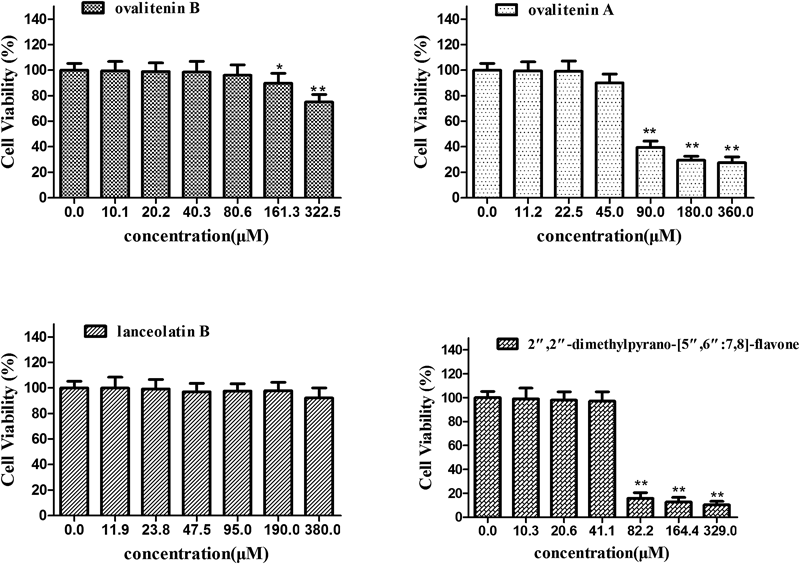
Cells were pretreated with indicated concentrations of four compounds or were left untreated for 24 h. Data is the average of three independent experiments and expressed as mean±S.E.M. * p<0.05, ** p<0.01, compared with vehicle control.
In order to induce NO synthesis, RAW264.7 macrophages were stimulated by treatment with LPS. The accumulation of nitrite as a stable product of NO metabolism was chosen to estimate the production of NO using the Griess reagent. NO production induced by LPS (1 µg/mL) in RAW264.7 macrophages was increased significantly (p<0.01) when compared with that in the control group. Ovalitenin B, ovalitenin A, lanceolatin B and 2″,2″-dimethylpyrano-[5″,6″:7,8]-flavone significantly (p<0.01 or p<0.05) decreased the nitrite accumulation in LPS-stimulated RAW264.7 cells in a concentration dependent manner (Fig. 6). The reduction in NO synthesis elicited by Lanceolatin B, with an IC50 value of 189.79±3.62 µM, was comparable to that seen with the positive control indomethacin, a commonly used antiinflammatory drug, with an IC50 value of 150.58±2.91 µM (data not shown).
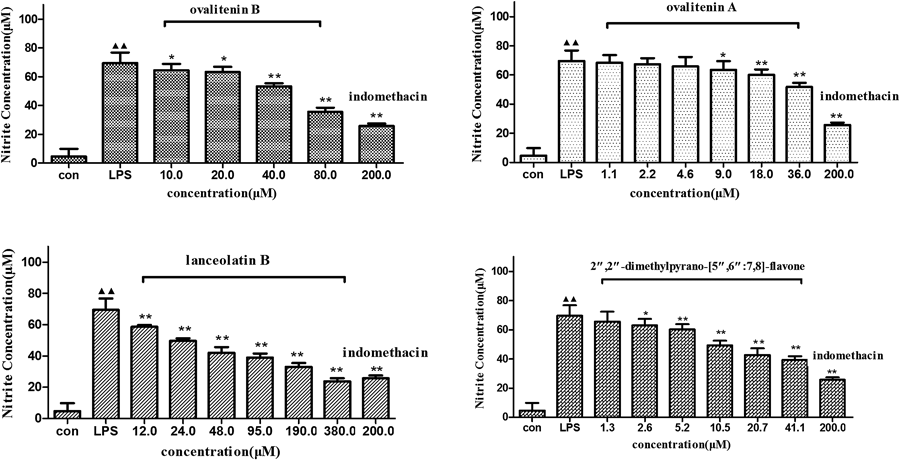
Macrophages were pretreated with LPS (1 µg/mL), with or without indomethacin (200 µM) and various concentrations of ovalitenin B, ovalitenin A, lanceolatin B and 2″,2″-dimethylpyrano-[5″,6″:7,8]-flavone for 24 h. Each data is an average of 3 independent experiments. Data are expressed as mean±S.E.M. ▲▲ p<0.01, compared with normal control group; * p<0.05, ** p<0.01, compared with LPS group.
Lanceolatin B, which exhibited the greatest suppression of NO synthesis, was selected as the best compound to evaluate the effect of components of EMP on the production of proinflammatory cytokines, IL-1β, IL-6 and TNF-α. Using ELISA assays, the levels of IL-1β, IL-6 and TNF-α in the cell supernatant were found to be markedly increased after LPS (1 µg/mL) stimulation. Treatment with lanceolatin B produced a dose-dependent inhibition of IL-1β (Fig. 7A), IL-6 (Fig. 7B), and TNF-α (Fig. 7C) production (p<0.01).
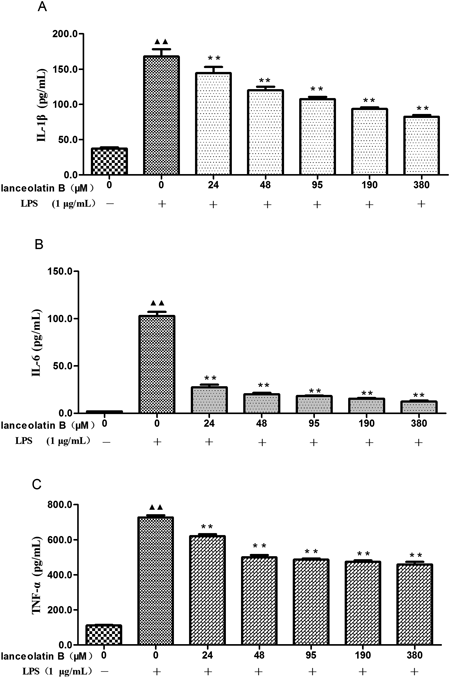
Macrophages were stimulated with LPS (1 µg/mL) in the presence of Lanceolatin B (final concentration 0, 24, 48, 95, 190, 380 µM) for 24 h. The concentration of cytokines (A: IL-1β, B: IL-6, and C: TNF-α) in the supernatants was determined by ELISA. Each data is an average of 3 independent experiments. Data are expressed as mean±S.E.M. ▲▲ p<0.01, compared with normal control group; ** p<0.01, compared with LPS group.
The effect of lanceolatin B on LPS-stimulated expression of COX-2, iNOS, NF-κB and p-IκBα in RAW264.7 macrophages was also determined to further explore the antiinflammatory mechanism. A Western blot experiment showed that, COX-2, iNOS, NF-κB and p-IκBα expression was upregulated in LPS-stimulated RAW264.7 macrophages (p<0.01), however, the COX-2, iNOS, NF-κB and p-IκBα expression levels after treatment with Lanceolatin B were significantly (p<0.01 or p<0.05) reduced in a dose-dependent manner (Fig. 8).

(A) Macrophages were stimulated with LPS (1 µg/mL) in the presence of lanceolatin B (final concentration 0, 95, 190, 380 µM) for 24 h. The protein levels of COX-2 and iNOS were determined by Western-blot analysis. β-Actin was used as a loading control. (B) RAW264.7 macrophages were incubated with lanceolatin B (final concentration 0, 95, 190, 380 µM) for 24 h followed by stimulation with LPS (1 µg/mL) for 15 min. The protein levels of NFκB and p-IκBα were determined by Western-blot analysis. β-Actin was used as a loading control. Each data is an average of 3 independent experiments. Data are expressed as mean±S.E.M. ▲▲ p<0.01, compared with normal control group; * p<0.05, ** p<0.01, compared with LPS group.
The present study reveals, for the first time, that oral administration of EMP produced strong antiinflammatory and analgesic activities in the xylene-induced ear edema model, the writhing test and the hot plate test. Additionally, individual compounds isolated from EMP reduced the level of inflammatory mediators produced by LPS-stimulated RAW264.7 macrophages.
Xylene-induced ear edema is an established animal model used for screening antiinflammatory agents. Xylene-induced ear edema is an acute and highly reproducible inflammatory response characterized by fluid accumulation and edema. Neurogenous swelling induced by xylene is partially related to substance p, and is commonly used for evaluating vascular permeability.22) EMP was shown to significantly reduce xylene-induced ear edema, indicating a strong antiinflammatory activity in vivo.
The acetic acid-induced writhing test has long been used as a screening tool to assess antiinflammatory and analgesic activities, and is considered to be a model for visceral inflammatory pain. It is generally accepted that acetic acid acts by increasing endogenous mediators, such as prostaglandin and histamine, in peritoneal fluids, leading to stimulation of nociceptive neurons.17) The abdominal constriction response is postulated to be induced by the activation of local peritoneal receptors.23) Administration of EMP reduced both the latency time and the number of acetic acid-induced abdominal constrictions, indicating analgesic activity.
To investigate the potential for central analgesic activity of EMP, a hot plate test was performed. Pain induced in the hot plate test was inhibited to a significant extent by the administration of EMP. This result, taken together with the analgesic activity of EMP in the writhing test, suggests both a peripheral and a central component to the analgesic activity of EMP.
LPS stimulation of RAW264.7 macrophages activates several intracellular signaling pathways which, in turn, lead to the activation of many transcription factors, including NF-κB. In unstimulated state, NF-κB is stabilized in cytosol by IκBα which is an inhibitory subunit. Once stimulated, IκBα becomes phosphorylated and this triggers its proteolytic degradation, leading to the activation and translocation of NF-κB to nucleus.24) NF-κB is a pleiotropic regulator of various genes encoding inflammatory mediators, such as proinflammatory cytokines (IL-1β, IL-6, TNF-α), iNOS and COX-2.17,25,26) The roles of these inflammatory mediators in the pathogenesis of various inflammatory conditions and pain have been firmly established.27,28) The antiinflammatory and analgesic activities of a particular drug can therefore be evaluated by detecting the expression of NF-κB, p-IκBα and the subsequent release of downstream mediators. Our data showed that lanceolatin B, a compound isolated from EMP, dose-dependently inhibited the phosphorylation of IκBα and expression of NF-κB, iNOS and COX-2 in RAW264.7 cells. Moreover, the levels of IL-1β, IL-6 and TNF-α in RAW264.7 cells were markedly reduced by lanceolatin B treatment. These findings suggested that the antiinflammatory properties of lanceolatin B were probably due to the inhibition of NF-κB and the suppression of IκBα phosphorylation, which then inhibited the subsequent release of downstream mediators.
The plant M. pulchra was commonly used in folk medicine for the management of inflammation, however, no side effects had been reported. Therefore, we suppose that the ethanol extract and lanceolatin B from M. pulchra might carry with less side effects, which is superior to that of the currently used drugs, and further research is required to confirm this hypothesis.
In conclusion, the results presented herein strongly suggest that EMP possesses antiinflammatory and analgesic activities, supporting the use of this plant species in folk medicine to treat pain and inflammation. Lanceolatin B, which shows the greatest inhibition of NO synthesis among the compounds tested, seems to possess strong antiinflammatory activity in vitro, acting through inhibition of IL-1β, IL-6, TNF-α, COX-2, iNOS, NF-κB and p-IκBα. Lanceolatin B may have therapeutic potential for the treatment of inflammatory diseases.
This study was supported by a grant from the Program for Innovative Research Team in IMPLAD (PIRTI).
The authors declare no conflict of interest.