Abstract
Protein kinase CK2 (“casein kinase II”) is a protein serine/threonine kinase that plays critical roles in biological processes such as cell growth, cell cycle progression, and apoptosis. So far, we have identified that one catalytic isozyme of CK2, CK2α, is over-expressed in the kidney during the progression of glomerulonephritis (GN). Moreover, we have shown that in vivo inhibition of CK2 by administration of CK2 inhibitors was effective in the treatment of experimental GN. Hence the development of potent CK2 inhibitors should be considered in therapeutic strategies for GN. In the present study we identified compound 13, a pyrazine derivative, as a potent CK2 inhibitor. By performing enzyme kinetics analysis in vitro, we characterized the inhibition of compound 13 toward each CK2 catalytic isozyme. Furthermore, in vivo, we demonstrated that compound 13 is effective in attenuating proteinuria, decreasing the enhanced level of blood urea nitrogen and serum creatinine, and ameliorating glomerular crescent formation in an experimental GN rat model. On the other hand, cellular apoptosis was detected in the rat testis following administration of compound 13. This study provides clues for new strategies for developing applicable compounds into CK2-targeted GN treatments.
Protein kinase CK2 (formerly called “casein kinase 2”) is a serine/threonine kinase ubiquitously distributed in eukaryotic organisms. CK2 phosphorylates more than 300 proteins,1) and its roles in cells are various, including cell proliferation, cell growth, cell division, and cell apoptosis.2) The CK2 holoenzyme in mammals exists predominantly as a heterotetramer composed of two catalytic subunits (α and/or α′) and two regulatory subunits (β). The two catalytic isozymes of CK2, CK2α and CK2α′, have been well characterized. CK2α and CK2α′ exhibit approximately 90% identity in their catalytic domains, even though they arise from different chromosomes.2) Although they may display similar enzymatic properties (including turnover rates and substrate specificity) in vitro, the two isozymes may play different roles in vivo, since CK2α is widely distributed in various tissues, whereas CK2α′ is mainly expressed in the brain and testis.3) In a report, the deficiency of CK2α in mice caused embryonic lethality, while the knockout of CK2α′ in mice induced spermatocytes to undergo apoptosis and the surviving spermatozoa to exhibit abnormal morphology.4) Hence, CK2α and CK2α′ may have distinct functions.
We have previously identified CK2α, but not CK2α′, as a glomerulonephritis (GN)-related gene using cDNA microarray analysis.5) In the same study, we observed that administration of either antisense oligodeoxynucleotide against CK2α or low molecular weight CK2 inhibitors effectively prevented the progression of renal dysfunction. Based on this background, we focused on developing novel CK2 inhibitors as a therapeutic agent for GN. We have synthesized a series of pyrazine derivatives related to the protein kinase inhibitors.6) Among the synthesized compounds, compound 13 showed the highest inhibitory activity in both enzymatic and cell-based CK2 inhibition assays. In that previous study, we preliminarily showed the inhibitory activity of compound 13 for CK2 against other protein kinases and its in vivo efficacy in the anti-glomerular basement membrane (anti-GBM GN) rat model. In the study herein, we examined the potency of compound 13 toward each CK2 catalytic isozyme for the first time. Moreover, physiologically and histologically, we investigated the effects of compound 13 on anti-GBM GN rat model in vivo. We demonstrated the significant antinephritic effect following administration of compound 13 in more detail than the previous study,6) while we also detected enhanced testicular apoptosis in the drug-treated experiment GN rat model. In summary, we provide an important suggestion for optimizing the targeting of CK2 inhibition for the therapy of various diseases including GN.
MATERIALS AND METHODS
AnimalsThe experimental protocol for this study was reviewed and approved by the Animal Care Committee of Kyoto University. Seven-week-old male Wistar-Kyoto (WKY) rats weighing 200 g were purchased from Charles River Japan (Atsugi, Kanagawa, Japan) and used in all the experiments. Animals were housed in a constant temperature room with a 12-h dark/12-h light cycle. The general condition and body weight of the rats were observed over the course of the experiments.
Isolation of GlomeruliGlomeruli were isolated from the renal cortices of the rats using the differential graded sieving method, which yields glomeruli with a purity greater than 90%.7) Briefly, kidneys were ground gently through 400-µm and then 200-µm stainless steel sieves. The obtained fraction was washed with phosphate buffered saline (PBS) on a 90-µm mesh. The supernatant was then centrifuged at 905×g for 5 min at 4°C (MX-300; TOMY, Tokyo, Japan).
Anti-GBM GNTo prepare anti-GBM antibodies, GBM antigen from rats was prepared from isolated glomeruli in accordance with the Krakower method.8) Five albino rabbits were immunized subcutaneously with GBM antigen emulsified with Freund’s complete adjuvant (Nihon Becton Dickinson, Tokyo, Japan). Three booster injections of the same antigen were given at two-week intervals. Four days after the final booster, blood was collected from the carotid artery under anesthesia. Anti-GBM sera were decomplemented by heating at 56°C for 30 min and absorbed with freshly harvested rat erythrocytes. Twenty-four WKY rats were divided into four groups of six rats (Control, anti-GBM GN, compound 13-treated anti-GBM GN and emodin-treated anti-GBM GN). The rats assigned to the GN groups were injected under anesthesia in the dorsal tail vein with 3 mL/kg anti-GBM serum that had been diluted 10-fold with saline. The day of the injection was defined as day 0. The rats assigned to the control groups were injected intravenously with 3.0 mL/kg normal nonimmune rabbit serum to allow comparison with rats in the GN groups.
Drug TreatmentThe CK2 inhibitor 3-methyl-1,6,8-trihydroxyanthraquinone (emodin; Sigma) prepared as a 25 mg/mL solution in dimethyl sulfoxide (DMSO), diluted to 1 : 10 with a 0.5% carboxymethylcellulose (CMC)-saline solution was administered intraperitoneally at 20 mg/kg and 2-[1-[6-[6-(cyclopentylamino)indazol-1-yl]pyrazin-2-yl]pyrrol-3-yl]acetic acid (compound 13; synthesized by Toray Industries, Co., Ltd.) dissolved in saline (2 mg/mL) was administered intravenously at 2.5 mg/kg or 5 mg/kg once a day after injection of the anti-GBM serum from day 1 to day 7 or day 14 at which time they were sacrificed. At the end of the study, the rats were anesthetized, their blood was collected by cardiac puncture, and their organs were collected.
Proteinuria and Serum Creatinine (SCr) DeterminationFor analysis of proteinuria, the rats were housed individually in metabolic cages for the 24-h urine collection. Urinary protein was measured by SRL, Inc. (Tokyo, Japan) using the Biuret method. Levels of blood urea nitrogen (BUN) and SCr were measured by SRL, Inc. (Tokyo) using an automated analyzer (Hitachi, Tokyo, Japan).
Histological Evaluation of Renal TissueKidney tissues from each animal were fixed in 10% neutral-buffered formalin (pH 7.4) and embedded in paraffin. Sections (4 µm) were then subjected to periodic acid-Schiff (PAS) staining, which were evaluated quantitatively by counting the total number of glomerular cells and the total number of glomeruli with mesangiolysis and capillary ballooning, and measuring areas of 20 randomly selected glomerular cross-sections with NIH Image (NIH, Bethesda, MD, U.S.A.). Glomerular crescent formation was assessed in a blinded manner. Crescent formation was defined as the presence of at least 2 non-tubular cell layers observed in the Bowman’s space.9) To describe the degree of crescent formation quantitatively, the crescentic score was used (number of glomeruli with crescent formation/30 glomeruli).
Terminal Deoxynucleotidyl Transferase-Mediated Deoxyuridine Triphosphate Nick-End Labeling (TUNEL) StainingTUNEL analysis of testis was performed using the Deadend™ Colorimetric TUNEL System (Promega Corp., Madison, WI, U.S.A.) according to the manufacturer’s protocol. Briefly, the deparaffinized sections were fixed in 4% paraformaldehyde solution for 15 min at room temperature, rinsed in PBS for 5 min 2 times, and permeabilized by immersing the sections in proteinase K for 30 min at room temperature. Sections were incubated with terminal deoxynucleotidyl transferase (TdT) reaction mixture (Equilibration buffer 98 µL, Biotinylated Nucleotide Mix 1 µL and recombinant TdT Enzyme 1 µL) at 37°C for 60 min, rinsed with saline sodium citrate for 15 min 2 times and PBS for 5 min 2 times. Sections were fixed in 0.3% hydrogen peroxide for 5 min to stop the reaction. Sections were washed by 0.2% Streptavidin-horseradish peroxidase solution (diluted with PBS) for 30 min at room temperature and then stained with Diaminobenzidine. The average number of TUNEL-positive cells in a testis section was determined by enumerating the number of TUNEL-positive cells per seminiferous tubule in each section. The TUNEL-positive cells were counted in a blinded manner.
Microarray Data AnalysisThe microarray data obtained from Gene Expression Omnibus (GEO; GSE8978) were examined and visualized using R/Bioconductor.10) The selected expression profiles were obtained from the whole testis of rats. Only probe sets with normalized signals >20 were defined as expressed and selected for analysis. Microarray analysis using Rat Genome 230 2.0 arrays identified a total of 16971 probe sets that recognized transcripts. The probe sets representing the CK2 subunits were 1384339_s_at and 1387170_at for CK2α, 1371326_at for CK2α′, and 1387108_at for CK2β. Values were represented as mean±standard error (S.E.) of the MAS5.0 signal intensity.
In Vitro CK2 Kinase AssayCK2 catalytic isozymes were prepared as described previously.11) The CK2 isozyme kinase reaction was performed in 15 µL of reaction mix that contained 20 mM 3-(N-morpholino)propanesulfonic acid (MOPS) pH 7.2, 25 mM β-glycerol phosphate, 5 mM ethylene glycol bis(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 1 mM sodium orthovanadate, 1 mM dithiothreitol, 13.5 mM MgCl2, 0.4 µM PKA inhibitor cocktail, 0.2 mM CK2 substrate peptide (RRRDDDSDDD), 8.5 mUnit CK2 isozyme, 0.09 mM ATP, 2.2 nM [γ-32P]ATP, 0.6 µL of inhibitor (emodin, compound 13 and hematein, which was purchased from Fluka (Sigma-Aldrich, Buchs, Switzerland, Cat. No. 51230)) or 4% DMSO (Nacalai Tesque, Kyoto, Japan) vehicle as a control. One unit of kinase activity is defined as the amount of enzyme that will catalyze the transfer of 1 nmol of phosphate to RRRDDDSDDD per min at 37°C. After incubation at 37°C for 10 min, the reaction was terminated by the addition of 10 µL of 40% trichloroacetic acid. Aliquots of 5 µL of the reaction mix were transferred to a 96-well P81 UniFilter plate (Whatman, Kent, U.K.), and each well was washed with 0.2 mL of 0.75% phosphoric acid solution 20 times. After incubation for 30 min in 0.02 mL of Microscinti-0 (PerkinElmer, Inc., CT, U.S.A.), residual radioactivity was measured using a TOP count NXT Scintillation and Luminescence Counter (PerkinElmer, Inc.). The type of enzyme inhibition was determined by the Lineweaver–Burk plot and its secondary plot.
Statistical AnalysisValues are represented as means± S.E. For the in vivo experiment, statistical analysis of differences between the groups was performed by ANOVA and subsequent t-testing with Bonferroni correction for multiple comparisons. Differences at p<0.05 were considered statistically significant.
RESULTS
Compound 13 Is a Potent CK2 InhibitorTo evaluate the potency of compound 13 to CK2α and CK2α′, we investigated the in vitro kinase activities of the two CK2 catalytic isozymes in the presence of compound 13. To better understand the inhibitory activities of compound 13, we also included two well-known CK2 inhibitors, emodin and hematein,5,12) in the kinase assay for comparison. The IC50 value of compound 13 towards CK2α and CK2α′ are 0.068 µM and 0.059 µM in the presence of 95 µM ATP, respectively (Table 1). This suggests that compound 13 is potent for both CK2 catalytic isozymes. A kinetics study was further performed by using Lineweaver–Burk plots, which is a graphical method for determining the dissociation constants of the binary enzyme–inhibitor complex (Ki) and the ternary enzyme-substrate–inhibitor complex (Ki′), and indicating the type of inhibition. The Lineweaver–Burk plots for both compound 13 and emodin showed non-parallel lines intersecting at the Y-axis, indicating an ATP-competitive inhibition of either CK2α or CK2α′ (Figs. 1A, B), which is in agreement with our previous study,13) while those for hematein showed non-parallel lines intersecting in the second quadrant indicating a mixed inhibition of both CK2 catalytic isozymes (Fig. 1C). From the secondary plots of the slopes or intercepts obtained from the Lineweaver–Burk plots, the apparent dissociation constants for the three compounds were calculated. The results of the calculation were shown as Ki for the slopes and Ki′ for the Y-axis intercepts in Table 1. The kinetic assays in our study showed that compound 13 and emodin are ATP-competitive inhibitors toward CK2 with no selectivity to either CK2α or CK2α′, whereas hematein has a partial non-competitive mixed type inhibition toward CK2 with a selectivity to CK2α′.
Table 1. Evaluation of the CK2 Inhibitory Activities of the Compounds
| Compound | Structures | CK2α | CK2α′ |
|---|
| IC50a) | Ki | Ki′ | IC50a) | Ki | Ki′ |
|---|
| Emodin |  | 12 | 15 | 2.7 | 2.7 | 66 | 2.9×103 |
| Compound 13 |  | 0.068 | 0.0087 | — | 0.059 | 0.010 | — |
| Hematein |  | 2.8 | 0.90 | 0.13 | 0.040 | 0.21 | 0.21 |
a) Inhibition values (µM) were determined by the CK2 kinase assay. Ki: a reversible binding of the compound to CK2α or CK2α′; Ki′: a reversible binding of the compound to CK2α-substrate complex or CK2α′-substrate complex.
Since we have demonstrated that administration of CK2 inhibitors ameliorated the progression of GN,5) we examined the in vivo effects of compound 13 on experimental GN. As with our previous study,5) WKY rats were injected with anti-GBM antibodies to induce experimental anti-GBM GN. In contrast to the control rats, the anti-GBM antibody-injected rats developed severe proteinuria, with the level of urinary protein excretion increased almost 5-fold at day 7 (Fig. 2A). Administration of emodin and compound 13 (2.5 mg/kg or 5 mg/kg) in the anti-GBM antibody-injected rats ameliorated the development of proteinuria significantly (Fig. 2A). In addition, the elevated BUN were significantly reduced by compound 13 (5 mg/kg) and emodin treatment and plasma creatinine levels was decreased in compound 13 (2.5, 5 mg/kg) and emodin (Fig. 2A). Hence, for the 14-d experiment, we administered 5 mg/kg compound 13 and found that the enhanced proteinuia, BUN, and SCr in anti-GBM GN rat at day 14 was attenuated by compound 13 (5 mg/kg) and emodin significantly (Fig. 2B).
We examined the effects of treatment with compound 13 or emodin on renal histology by PAS staining. The most prominent change in the anti-GBM GN rats was severe glomerular crescent formation as compared with the control rats (Fig. 2C). The degree of crescent formation was lower in the emodin and compound 13-treated rats than in rats treated with the vehicle (Fig. 2C). The crescentic score was highest in the anti-GBM GN rats, and was reduced significantly by the treatment with emodin or compound 13 (Fig. 2D).
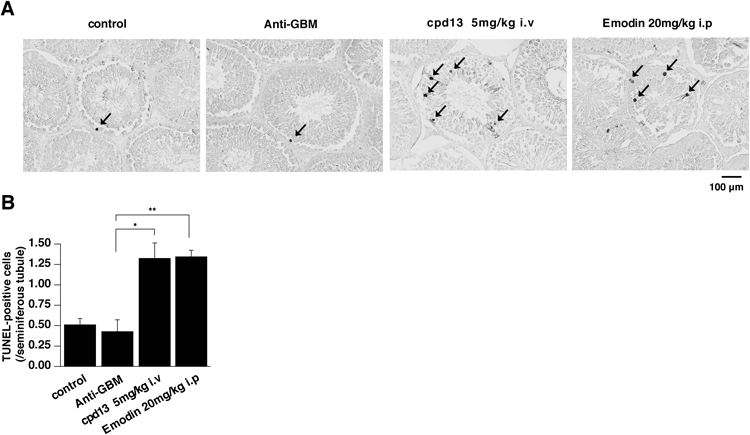
Compound 13 Has a Toxicological Effect on the Testis Tissue in VivoAs emodin is reported to show testicular toxicity,14) we examined the toxicological effect of compound 13 on testis by performing TUNEL analysis as compared with emodin. As shown in Fig. 3A, few TUNEL-positive cells, which indicate cells undergoing apoptosis, were observed in the testis tissues of the anti-GBM GN rats and the control rats. On the other hand, we observed an increased number of TUNEL-positive cells in the testis tissue of the emodin-treated and compound 13-treated anti-GBM GN rats (Fig. 3A). By counting the number of TUNEL-positive cells per seminiferous tubule, we revealed that testicular apoptosis was increased significantly in the experimental groups treated with emodin or compound 13 (Fig. 3B).
Since compound 13 is a selective inhibitor for CK2 against other protein kinases6) and cellular apoptosis was increased significantly by knockout of CK2α′,15) we investigated the expression of each CK2 subunit in rat testis tissue using public domain microarray data obtained from GEO (GSE8978).16) Predictably, as shown in Table 2, CK2α′ were highly expressed in the testis, while CK2α was expressed at the lowest detectable level.
Table 2. The Distinct Expression Patterns of CK2 Subunits in Whole Rat Testis (GSE8798)
| Gene name | Gene symbol | Probe ID | Value (mean, n=5, MAS 5.0 signal intensity) |
|---|
| CK2α | Csnk2a1 | 1384339_s_at | 28.5±5.90 |
| | 1387170_at | 28.7±2.90 |
| CK2α′ | Csnk2a2 | 1371326_at | 460±14.7 |
| CK2β | Csnk2b | 1387108_at | 3.41×103±198 |
DISCUSSION
CK2 has been studied for many years as a multifunctional and pleiotropic protein kinase.17) It was reported to be implicated in a series of physiological and pathological processes, including various cancers,18) bone formation,19) mouse globozoospermia,15) embryonic morphogenesis,20) and chronic intestinal inammation.21) We have previously identified CK2 as a GN-related gene and indicated that CK2 could be a therapeutic target for treating GN.5) In that study, we demonstrated that administration of CK2 inhibitors in experimental GN model rats ameliorates the renal dysfunction and histological progression. Recently, in our another study, we have provided a mechanistic clue for the understanding of experimental crescentic GN and the pharmacological effects of a CK2 inhibitor and other treatment by microarray analysis.22) The results provided further insight on the possible relationship between GN and CK2. To develop CK2 inhibitors as therapeutic agents for GN, Fuchi et al. had synthesized compound 13 based on the structure of 2,6-disubstituted pyrazines.6) They had identified compound 13 as a potent and selective CK2 inhibitor and had found that compound 13 attenuated proteinuria derived from the anti-GBM antibody-induced renal dysfunction. To further investigate the antinephritic effect of compound 13, we examined the physiological and histological effects on the experimental GN rat model exposed to compound 13 in the present study. We found that compound 13 has a significant antinephritic effect even at a lower dose of 5 mg/kg than in the previous case.6)
Moreover, in the present study, enhanced testicular apoptosis was detected following administration of compound 13 (Fig. 3). Since compound 13 is a selective CK2 inhibitor,6) we can suggest that CK2 may be the key molecule in this enhanced testicular apoptosis. Previously, administration of emodin, a less selective CK2 inhibitor, is also reported to show testicular toxicity.14) In this study, by using the more selective CK2 inhibitor, we demonstrated that this testicular toxicity may be related to CK2 inhibition. Furthermore, we confirmed a higher expression pattern of CK2α′ than CK2α in the rat testis tissue bymicroarray data analysis (Table 2). As compound 13 is potent for both CK2α and CK2α′ (Fig. 1B), we can indicate that CK2α′ may play an important role in the testicular toxicity following administration of compound 13. Previous studies have reported that CK2α′ is highly-expressed in the testis and knockout of CK2α′ induces massive spermatocytes to undergo apoptosis.15,16) Hence, our results raises the possibility that the inhibition of CK2α′, but not CK2α, by CK2 inhibitors induced these toxicological effects on testis tissue. Considering the overexpression of CK2α in the progression of GN5) and the potential testicular toxicity by inhibition of CK2α′, a CK2α-specific inhibitor may be suitable for further investigation for the treatment of GN with possible less testicular toxicity.
During the development of CK2α-specific inhibitors, CK2 enzymatic analysis from our group and another group on hematein,12) has led us to discover that hematein is a partial ATP non-competitive mixed type inhibitor toward CK2 (Fig. 1C). Moreover, our group revealed that hematein has a greater selectivity for CK2α′ with a lower IC50 value (Table 1). So far, although a number of ATP non-competitive inhibitors toward CK2 have been identified in vitro, their inhibitory activities in vivo were low or have not been elucidated.23,24) In consideration of the high intensity of ATP in cells, compounds with an ATP-noncompetitive type inhibition of CK2α or an ATP-competitive type inhibition of CK2α′ may exhibit the desired antinephritic effects with less testicular toxicity. According to the structure of hematein, we can provide a clue for the development of ATP-noncompetitive CK2 inhibitors with a CK2α selectivity.
In conclusion, the results of the present study determined the inhibitory acitivity of a potent CK2 inhibitor toward the CK2 catalytic isozymes, identified its inhibition pattern, verified its preventive effects on the progression of GN, and indicated the possibility that the toxicological effect on testis tissue by administration of the CK2 inhibitor may be related to the inhibition of CK2α′. Hence, considering the overexpression of CK2α during the progression of GN,5) the development of a CK2α-specific inhibitor or an ATP-noncompetitive inhibitor of CK2α may provide a new strategy in developing applicable compounds into CK2α-targeted GN therapies.
Acknowledgments
This study was supported by Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO; to G.T.), Grants-in-Aid for Scientific Research and Targeted Protein Research Program from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) (to G.T.), a research grant from the Scientific Fund of the (to A.H.). J. S. is supported by the Otsuka Toshimi Scholarship Foundation. Thanks to Ms. Ayako Hirata for the manuscript preparation.
Conflict of Interest
Dr. Masateru Yamada, Dr. Nobuhiro Fuchi, Dr. Keiyu Oshida and Dr. Yohei Miyamoto are employees of Toray Industries Inc. Dr. Yoshinori Takei belongs to anendowed chair faculty from Toray Industries, Inc. at Kyoto University. There is no other conflict of interest.
REFERENCES
- 1) Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J., 17, 349–368 (2003).
- 2) Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem. J., 369, 1–15 (2003).
- 3) Alvarado-Díaz CP, Tapia JC, Antonelli M, Moreno RD. Differential localization of alpha′ and beta subunits of protein kinase CK2 during rat spermatogenesis. Cell Tissue Res., 338, 139–149 (2009).
- 4) St-Denis NA, Litchfield DW. Protein kinase CK2 in health and disease: From birth to death: the role of protein kinase CK2 in the regulation of cell proliferation and survival. Cell. Mol. Life Sci., 66, 1817–1829 (2009).
- 5) Yamada M, Katsuma S, Adachi T, Hirasawa A, Shiojima S, Kadowaki T, Okuno Y, Koshimizu TA, Fujii S, Sekiya Y, Miyamoto Y, Tamura M, Yumura W, Nihei H, Kobayashi M, Tsujimoto G. Inhibition of protein kinase CK2 prevents the progression of glomerulonephritis. Proc. Natl. Acad. Sci. U.S.A., 102, 7736–7741 (2005).
- 6) Fuchi N, Iura Y, Kaneko H, Nitta A, Suyama K, Ueda H, Yamaguchi S, Nishimura K, Fujii S, Sekiya Y, Yamada M, Takahashi T. Discovery and structure–activity relationship of 2,6-disubstituted pyrazines, potent and selective inhibitors of protein kinase CK2. Bioorg. Med. Chem. Lett., 22, 4358–4361 (2012).
- 7) Liu N, Makino T, Nogaki F, Kusano H, Suyama K, Muso E, Honda G, Kita T, Ono T. Coagulation in the mesangial area promotes ECM accumulation through factor V expression in MsPGN in rats. Am. J. Physiol. Renal Physiol., 287, F612–F620 (2004).
- 8) Yamada M, Sasaki R, Sato N, Suzuki M, Tamura M, Matsushita T, Kurumatani H. Amelioration by beraprost sodium, a prostacyclin analogue, of established renal dysfunction in rat glomerulonephritis model. Eur. J. Pharmacol., 449, 167–176 (2002).
- 9) Ophascharoensuk V, Pippin JW, Gordon KL, Shankland SJ, Couser WG, Johnson RJ. Role of intrinsic renal cells versus infiltrating cells in glomerular crescent formation. Kidney Int., 54, 416–425 (1998).
- 10) Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol., 5, R80 (2004).
- 11) Nakaniwa T, Kinoshita T, Sekiguchi Y, Tada T, Nakanishi I, Kitaura K, Suzuki Y, Ohno H, Hirasawa A, Tsujimoto G. Structure of human protein kinase CK2 alpha 2 with a potent indazole-derivative inhibitor. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun., 65, 75–79 (2009).
- 12) Hung MS, Xu Z, Lin YC, Mao JH, Yang CT, Chang PJ, Jablons DM, You L. Identification of hematein as a novel inhibitor of protein kinase CK2 from a natural product library. BMC Cancer, 9, 135 (2009).
- 13) Kinoshita T, Sekiguchi Y, Fukada H, Nakaniwa T, Tada T, Nakamura S, Kitaura K, Ohno H, Suzuki Y, Hirasawa A, Nakanishi I, Tsujimoto G. A detailed thermodynamic profile of cyclopentyl and isopropyl derivatives binding to ck2 kinase. Mol. Cell. Biochem., 356, 97–105 (2011).
- 14) Oshida K, Hirakata M, Maeda A, Miyoshi T, Miyamoto Y. Toxicological effect of emodin in mouse testicular gene expression profile. J. Appl. Toxicol., 31, 790–800 (2011).
- 15) Xu X, Toselli PA, Russell LD, Seldin DC. Globozoospermia in mice lacking the casein kinase II alpha′ catalytic subunit. Nat. Genet., 23, 118–121 (1999).
- 16) Johnston DS, Wright WW, Dicandeloro P, Wilson E, Kopf GS, Jelinsky SA. Stage-specific gene expression is a fundamental characteristic of rat spermatogenic cells and Sertoli cells. Proc. Natl. Acad. Sci. U.S.A., 105, 8315–8320 (2008).
- 17) Filhol O, Cochet C. Protein kinase CK2 in health and disease: Cellular functions of protein kinase CK2: a dynamic affair. Cell. Mol. Life Sci., 66, 1830–1839 (2009).
- 18) Trembley JH, Chen Z, Unger G, Slaton J, Kren BT, Van Waes C, Ahmed K. Emergence of protein kinase CK2 as a key target in cancer therapy. Biofactors, 36, 187–195 (2010).
- 19) Bragdon B, Thinakaran S, Moseychuk O, Gurski L, Bonor J, Price C, Wang L, Beamer WG, Nohe A. Casein kinase 2 regulates in vivo bone formation through its interaction with bone morphogenetic protein receptor type Ia. Bone, 49, 944–954 (2011).
- 20) Dominguez I, Degano IR, Chea K, Cha J, Toselli P, Seldin DC. CK2 alpha is essential for embryonic morphogenesis. Mol. Cell. Biochem., 356, 209–216 (2011).
- 21) Koch S, Capaldo CT, Hilgarth RS, Fournier B, Parkos CA, Nusrat A. Protein kinase CK2 is a critical regulator of epithelial homeostasis in chronic intestinal inammation. Mucosal. Immunol., 6, 136–145 (2013).
- 22) Liu N, Shi J, Xiao Y, Yasue M, Takei Y, Sanefuji H, Tsujimoto G, Hirasawa A. Effects of a tricaprylin emulsion on anti-glomerular basement membrane glomerulonephritis in rats: in vivo and in silico studies. Biol. Pharm. Bull. 38, 1175–1184 (2015).
- 23) Prudent R, Cochet C. New protein kinase CK2 inhibitors: jumping out of the catalytic box. Chem. Biol., 16, 112–120 (2009).
- 24) Cozza G, Bortolato A, Moro S. How druggable is protein kinase CK2? Med. Res. Rev., 30, 419–462 (2010).