2015 Volume 38 Issue 9 Pages 1361-1367
2015 Volume 38 Issue 9 Pages 1361-1367
A major challenge in stem cell therapy for cardiac repair is how to obtain normally functioning stem cell-derived cardiomyocytes. We aim to address the effects of C-reactive protein (CRP) on the cardiac differentiation of embryonic stem (ES) cells. Immunostaining, Western blotting and electrophysiology were employed. A hundred fifty milligran/liters CRP significantly reduced the percentage of cardiomyocytes differentiated from mouse ES cells, while it may also promote sarcomere development compared to 30 mg/L CRP treatment. Further examination of the action potential (AP) in individual ES cell-derived cardiomyocytes showed that there exist three types of cardiomyocytes: artial-like (A-like), ventricular-like (V-like), and pacemaker-like (P-like). A hundred fifty milligran/liters CRP treatment decreased the P-like cardiomyocytes, whereas it increased the A-like. Such inhibitory effect and alteration were not significant at 30 mg/L CRP treatment. Moreover, 150 mg/L CRP significantly increased the APD90 (90% of duration of AP) and decreased the spontaneous firing rate of AP in P-like cells, while had little effect on other electrophysiological characteristics, including APA (AP amplitude) and MDP (maximum diastolic potential). This study revealed the effect of CRP on the cardiac differentiation of ES cells. It provides an in vitro pathological model and may be of importance to the future work of ES cell-based therapy in clinical applications and in vivo pathological studies.
Cardiac diseases are the leading cause of morbidity and mortality worldwide. Unlike some organs, the heart has pretty limited, if any, capacity for repair after injury. In particular, most adult cardiomyocytes are unable to divide and form new cardiomyocytes to replace those lost in cardiac diseases, such as in myocardial infarction, dilated cardiomyopathy, heart failure, and so forth. Traditional pharmacological management has limited efficacy and can not reverse or even prevent the progression of cardiac diseases.1) The ultimate approach of heart transplantation is costly and it excludes patients who are poor candidates for transplantation owing to their comorbidities, or for whom a donor organ is not available.
Stem cell therapy has gained more and more popularity for cardiac repair over the years since stem cells can differentiate into cardiomyocytes, endothelial cells, and other types of cells to replace those lost in cardiac diseases after being transplanted.1,2) Various types of stem cells have been studied in laboratory experiments and in clinical trials, including embryonic stem (ES) cells, mesenchymal stem cells (MSC), and endothelial progenitor cells (EPCs).1,2) ES cells are pluripotent and have the potential to differentiate into tissue derivatives of all three embryonic germ layers. Numerous studies and reports demonstrated that ES cell-derived cardiomyocytes improved cardiac function significantly after being transplanted into the impaired myocardium.1–4) MSCs, found in bone marrow, muscle, skin, and adipose tissues, can differentiate into any tissues of the mesenchymal origin.5) Several studies indicated that MSCs differentiated into cardiomyocytes in vivo, promoted capillary formation and cardiac function.6–9) EPCs are also derived from adult bone marrow, and can increase capillary formation by promoting vasculogenesis and angiogenesis, which in turn reduce collagen deposition and the apoptosis of cardiomyocytes.10) Despite significant advances in the past years in stem cell-based therapy in animal models and in clinical trials, there are still many obstacles needed to be solved before it can be fully applied, especially, how to obtain normally functioning stem cell-derived cardiomyocytes.1)
C-Reactive protein (CRP) is an annular, pentameric protein in the blood plasma and its level increases in response to inflammation.11,12) CRP plays an important role in the initiation and progression of different types of cardiovascular diseases.13–15) Patients with high CRP concentrations are more likely to develop atherothrombosis, stroke, myocardial infarction, and severe peripheral vascular diseases.16) In a rat model, its overexpression exacerbated ischemic necrosis and cerebral infarcts in the complement-mediated inflammation.17) Moreover, CRP has direct effects on stem cells. It impaired EPC antioxidant defenses, promoted EPC apoptosis, and suppressed the differentiation of EPCs into endothelial cells.18,19) However, the effect of CRP on ES cells is yet to be further researched. In the present study, we examined the cardiac differentiation of mouse ES cells with presence of different concentrations of CRP, and we further investigated the effect of CRP on the electrophysiological properties in the ES cell-derived cardiomyocytes.
Mouse embryonic fibroblasts (MEFs) were prepared from mouse embryos (13.5 D), cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Grand Island, NY, U.S.A.) supplemented with 10% fetal bovine serum (FBS, Hyclone, Rockville, MD, U.S.A.), and passaged every 3 d. The mouse ES cell line D3 (purchased from ATC C) were cultured either feeder-free or on a mitomycin-C (Sigma-Aldrich, St. Louis, MO, U.S.A.) treated MEF feeder layer in DMEM supplemented with 15% FBS, 2 mM L-glutamine (Gibco), 0.1 mM β-mercaptoethanol (Sigma-Aldrich), 1% non-essential amino acids (NEAA, Gibco), and 1000 U/mL leukemia inhibitory factor (LIF, Chemicon, Temecula, CA, U.S.A.). Upon 80–90% confluent, mouse ES cells were passaged with 0.25% trypsin–ethylenediaminetetraacetic acid (EDTA) (Hyclone). The cells were cultured at 37°C and 5% CO2. The care and use of animals in the experiments followed the guidelines approved by the Institutional Animal Care and Use Committee (IACUC) of Nanjing Medical University Affiliated WuXi Second Hospital. The IACUC committee members at Nanjing Medical University Affiliated WuXi Second Hospital approved this study. All efforts were made to minimize the number of animals used and their suffering.
Differentiation of Mouse Embryonic Stem Cells into CardiomyocytesMouse ES cells were seeded into a 6-well plate (0.1% gelatin coated) at a density of 10000 cells per well. Twenty four hours later, the culture medium was changed to conditioned medium (LG-DMEM supplemented with 10% FBS) with 10 µM 5-azacytidine (5-Aza) for differentiation. After induced by 5-Aza for 24 h, the cells were washed with phosphate buffered saline (PBS) solution and further cultured in the conditioned medium with or without 30 or 150 mg/L CRP treatment until D20. The medium was changed every 2 d during experiments.
ImmunofluorescenceFor immunofluorescence staining, after the cells were differentiated for 2 weeks post-plating (D21), cells were firstly fixed with 4% paraformaldehyde for 30 min at room temperature. After permeabilized with 0.1% Triton X-100 for 10 min and blocked with 5% bovine serum albumin (BSA)/PBS solution for 30 min, the cells were stained with primary antibody against Oct4 or α-actinin (Sigma-Aldrich), and 4′-6-diamidino-2-phenylindole (DAPI) (Sigma) in 5% BSA/PBS solution for 30 min in the dark. After 3washes with PBS solution (5 min a time), the cells were further incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Santa Cruz, TX, U.S.A.) for 30 min in the dark. Fluorescence images were acquired with a fluorescence microscope.
Western BlottingAs a routine method, Western blotting was performed as conducted in numerous previously published literatures. Cells were collected for Western blotting at D21. The antibodies used in the study were α-actinin (Sigma), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Abcam, MA, U.S.A.). Three independent experiments were conducted in the study.
Electrophysiological RecordingCells were subjected to electrophysiological recording at D21 (two weeks post-plating). Mouse ES cells were plated onto a coverslip for the recording chamber, and the action potential in cardiomyocytes were recorded with a whole-cell patch clamp equipped with an Axon 200B amplifier (Axon Instruments, NY, U.S.A.). The signal was digitized at 20 kHz, filtered at 2 kHz, and analyzed with PClamp 9.0 program. The patch pipette (2–4 MΩ resistance) was filled with an solution containing 50 mM potassium chloride (KCl), 60 mM K-aspartate, 1 mM MgCl2, 3 mM Na2ATP, 10 mM ethylene glycol bis(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), and 10 mM N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES). The pH was adjusted to 7.3 with potassium hydroxide (KOH). Only the cells with spontaneous beating were included in the following observation of action potentials and subsequent analysis.
Statistical AnalysisData was expressed as mean±standard error of the mean (S.E.M.). Statistical analysis between groups was performed by the one-way ANOVA test followed by a Tukey test. Statistical p values <0.05 were considered significant.
In order to determine the effect of CRP on the cardiac differentiation, mouse ES cells were firstly cultured in suspension for 6 d forming embryoid bodies (EBs) before being plated into a 6-well plate (0.1% gelatin coated) at day 7. 5-Aza was added into the culture medium for induction at day 8 for 24 h before being washed out. Further, the cells were cultured and treated with 30 mg/L or 150 mg/L CRP until the experiment was terminated at day 20 (Fig. 1A). The cell condition was checked with a microscope during culture and differentiation. Undifferentiated ES cells in suspension-culture formed EBs during differentiation (Fig. 1B). After being plated, ES cells adhered to the plate surface (Fig. 1C). Immunostaining against Oct4, a specific marker of ES cells, showed that the majority of the cells were Oct4-positive (Fig. 1D), confirming that the cells used were ES cells in the study.

(A) Experimental procedures for ES cell culturing in this study. Undifferentiated ES cells in suspension culture for 6 d (B) and in adherent culture after plating in day 8 (C). (D) Undifferentiated ES cells were characterized by immunostaining for their marker Oct4. Scale bar=100 µm.
Immunostaining was further used to study the effect of CRP on the cardiac differentiation of mouse ES cells. Immunostaining against α-actinin showed that the mouse ES cells differentiated into cardiomyocyte, as evidenced by the appearance of α-actinin positive cells (Fig. 2A) since α-actinin is a marker of cardiomyocytes. Of note, sarcomeres seem to be more well developed under 150 mg/L treatment compared with the condition of 30 mg/L CRP (Fig. 2A). When the ES cells were treated with 30 mg/L CRP, the percentage of α-actinin positive cells did not change, compared to that in the control (Figs. 2A, B). This observation was confirmed by Western blot analysis, in which they exhibited a similar optical density of α-actinin after normalized with the loading control GAPDH (Fig. 2B). However, 150 mg/L CRP treatment reduced the percentage of cardiomyocyte differentiated from ES cells, as illustrated by a noticeable decrease in α-actinin positive cells (Figs. 2A, B). Western blot analysis confirmed the inhibitory effect of 150 mg/L CRP (Fig. 2C), which was consistent with previous findings.18,19) The results were further confirmed by another marker, the antibody against sarcomeric myosin heavy chain, used to identify the cardiomyocytes differentiated from ES cells (data not shown). Together, a high level of CRP (150 mg/L), but not a moderate level (30 mg/L), reduced the number of cardiomyocytes differentiated from ES cells significantly, while may promote sarcomere development.

(A) The representative immunostaining images of differentiated ES cells against anti-α-actinin and the nucleus (DAPI) in control, 30 mg/L CRP and 150 mg/L CRP treatment groups. (B) The percentage of anti-α-actinin positive cell in control (n=385), 30 mg/L CRP (n=372) and 150 mg/L CRP (n=403) treatment groups. (C) Western blot analysis of the expression of α-actinin in these three groups. Scale bar=100 µm. Data were presented as mean±S.E.M. * p<0.05.
The action potential type in the differentiated cardiomyocytes was examined with a whole-cell patch clamp technique. Two weeks’ differentiation after plating, most of the cells exhibited spontaneous beating, which were included for the subsequent electrophysiological recordings and statistical analysis. For those cells without automaticity, they were excluded for the following experiments.
Generally, we define the cell types on the action potentials of the cells. First, the cells did not generate action potentials were considered to be undifferentiated stem cells. Those cells which were capable of firing actions potentials were further examined by the shape and properties of action potentials to define their types (pacemaker-like (P-like), atrial-like (A-like), ventricular-like (V-like)). We measured the maximum rate of rise of the action potential (dV/dtmax), the action potential duration (APD), action potential amplitude (APA) and phase 4 depolarization. P-like cardiomyocytes were characterized by their action potentials of prominent phase 4 depolarization, slow dV/dtmax and relatively smaller APA. For V-like and A-like cardiomyocytes, the action potentials have rapid dV/dtmax (nearly vertical shape in the rise phase of action potential). Furthermore, the action potentials of A-like cardiomyocytes usually exhibited more triangular shapes compared to those of P-like and V-like, while V-like cardiomyocytes were characterized by their action potentials of longer duration of APD and much larger APA compared to those of A-like and P-like.
The result showed that the differentiated cardiomyocytes exhibited three types of action potential: P-lik), A-like and V-like (Fig. 3A). Further statistical analysis showed that the percentage of the three types of cardiomyocytes did not change significantly with the presence of 30 mg/L CRP, compared to that in the control (Fig. 3B). Interestingly, 150 mg/L CRP treatment significantly decreased the P-like cell percentage and increased the A-like, but did not change the V-like (Fig. 3B). Together, it was demonstrated that a high dose of CRP (150 mg/L) influenced the cardiac differentiation of ES cells in terms of the action potential type in the ES cell-derived cardiomyocytes, while a low dose of CRP (30 mg/L) did not.
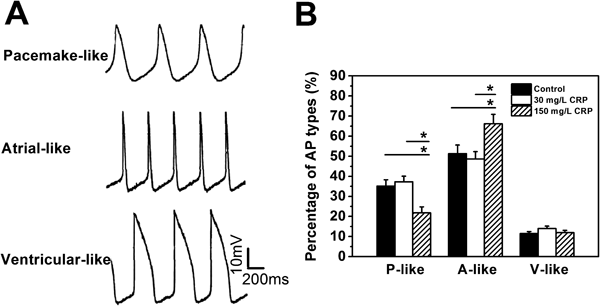
(A) The typical APs for three different kinds of cardiomyocytes differentiated from ES cells for 14 d, pacemaker-like cardiomyocytes (P-like), atrial-like cardiomyocytes (A-like) and ventricular-like cardiomyocytes (V-like). (B) The percentages of AP types in control, 30 mg/L CRP and 150 mg/L CRP treatment groups. Two-hundred fourty-six total cells were counted. Data were presented as mean±S.E.M. * p<0.05.
The detailed electrophysiological characteristics of the three types of cardiomyocytes were further examined, including the action potential duration at 90% repolarization (APD90), APA, and the maximum diastolic potential (MDP). The values of APD90, APA, and MDP did not change significantly with the presence of 30 mg/L or 150 mg/L CRP (Fig. 4) in the three types of cardiomyocytes, except for the APD90 in P-like cardiomyocytes which increased with the presence of 150 mg/L CRP (Fig. 4A). Similar results were observed in spontaneous firing rates of the action potentials, which were significantly decreased in P-like cardiomyocytes under 150 mg/L CRP treatment (Fig. 4D).

Data were presented as mean±S.E.M. * p<0.05.
The physiological concentration of CRP in the healthy human body is usually lower than 10 mg/L, and it increases significantly under pathological situations. For instance, higher levels are found in mild inflammation (10–40 mg/L), active inflammation (40–200 mg/L), and severe bacterial infections or burns (>200 mg/L).20) To simulate the mild and acute inflammatory concentrations in vivo, the effect of CRP on the cardiac differentiation of ES cells was investigated at 30 mg/L and 150 mg/L CRP respectively in the study. It would be of clinical significance in the treatment of human cardiac diseases with ES cell-based therapy.
In the research, the culture and induction of ES cells were well controlled. ES cells were firstly suspension-cultured forming EBs before being plated into a 0.1% gelatin-coated plate for adherent culture and induction with 5-Aza. The experiment demonstrated that ES cells had the ability to differentiate into cardiomyocytes properly. Worthy of mention, numerous induction protocols were reported, including optimized culture medium supplemented with different types of growth factors as well as with various compounds, such as PFT-α, 5-Aza, Ang-II, and BMP-2. 5-Aza was used in our study, as in many other previously published literatures.21–24) 5′-Aza is an inhibitor of DNA methylation, and is critical in the induction of new developmental pathways in cultured cells since cell differentiation involves stable and heritable changes, presumably of an epigenetic nature.24) Of note, the cardiomyocytes differentiated from ES cells for 16 d were used for further electrophysiological examinations (Figs. 3, 4) so that they were differentiated mature.
Immunostaining against the cardiomyocyte-specific protein of α-actinin confirmed that cardiomyocytes could be derived from ES cells (Fig. 2A). In the study, it was revealed that 150 mg/L CRP reduced the percentage of cardiomyocytes differentiated from mouse ES cells. With the presence of 150 mg/L CRP, 34% cells were cardiomyocytes, as compared to 55% in the control. However, such inhibitory effect was not significant at 30 mg/L CRP. However, the immunostaining result in Fig. 2A suggests 150 mg/L CRP may promote sarcomere development when compared to 30 mg/L CRP treatment. The differentiation process is very complicated and the underlying mechanisms are poorly understood. There are studies and reports demonstrating that many signaling pathways or compounds are involved in, such as STAT3, BMP, Wnt, FGF, PGC-1α, and reactive oxygen species.25–30) It was not surprising that the state of the signaling pathways or compounds during differentiation might be modulated by a strong extracellular stimulus of 150 mg/L CRP, but not by a weaker stimulus of 30 mg/L CRP. Nevertheless, the present study demonstrated the effect of 150 mg/L CRP on the cardiac differentiation of ES cells.
Based on the type of the action potential, including resting potential, upstroke velocity, amplitude, and duration, ES cell-derived cardiomyocytes are classified into three types: P-like, A-like and V-like).31) The P-like cardiomyocytes are characterized by a relatively depolarized resting membrane potential, while the A-like and V-like display a more stable and hyperpolarized resting potential. It was revealed that 150 mg/L CRP decreased the percentage of the P-like and increased the A-like, and did not change the V-like during differentiation. However, such alteration did not happen at 30 mg/L CRP. It suggested the differentiation of ES cells into different types of cardiomyocytes was altered by 150 mg/L CRP, but not by 30 mg/L CRP (mild inflammation). Also, previous studies suggest the characteristic pattern of action potential in ES-differentiated cardiomyocytes depends on the differentiation state.32) A hundred milligram/liters CRP treatment decreased the percentage of P-like action potentials while increased the percentage of A-like action potentials, suggesting its capability of accelerating the ES differentiation to the terminally differentiated cardiomyocytes, which is desired for the cell therapy to treat cardiovascular diseases.
Expression levels of voltage-gated ion channels on the cell membrane, such as Na+, Ca2+, K+, Cl− channels, determine the electrophysiological property of a cardiomyocyte. Their expression levels in a cardiomyocyte are reflected by the action potential duration at 90% of the repolarization (APD90), APA, MDP, etc. With the presence of CRP at 30 mg/L or even 150 mg/L, there was no significant change in APD90, APA, or MDP in an individual mouse ES cell-derived cardiomyocyte, compared to the control without the treatment of CRP. Of note, with the presence of 150 mg/L CRP, ADP90 in the P-like increased. Taken together, CRP had little effect on electrophysiological characteristics of the action potential in an individual differentiated cardiomyocyte during differentiation. It might suggest that CRP had little effect on the expression of voltage-gated ion channels on the cell membrane in the differentiated cardiomyocytes even under an acute inflammatory condition (150 mg/L CRP).
Especially, stem cells therapy is usually conducted under pathological conditions, if for clinical purpose. We think the biggest advantage of using CRP is that it can mimic the pathological condition of the cardiac diseases, especially in the concentrations we chose. So, it might provide an in vitro pathological model to study the fundamental questions of cardiac differentiation. In other words, the future work, especially on clinical applications or in in vivo pathological situation, should take into account the fact that CRP may have direct effects on ES cell differentiation to the cardiomyocytes and their electrophysiological features.
To summarize, mouse ES cells differentiated into cardiomyocytes properly in the present study. A hundred fifty milligram/liters CRP reduced the percentage of cardiomyocytes differentiated from mouse ES. Meanwhile, 150 mg/L CRP increased the A-like percentage and decreased the P-like percentage in the differentiated cardiomyocytes. However, such inhibitory effect or alteration was not significant or observed at 30 mg/L CRP. Furthermore, 30 mg/L or even 150 mg/L CRP did not change electrophysiological characteristics of the action potential in an individual ES cell-derived cardiomyocyte as reflected by no changes in APD90, APA, and MDP, except an increase in ADP90 decrease in spontaneous firing rate in the P-like (150 mg/L CRP). In short, the present study revealed the effect of CRP on the differentiation of ES cells into cardiomyocytes with mild (30 mg/L CRP) or acute (150 mg/L CRP) inflammation. It would be of significant importance in the treatment of cardiac diseases in the human being with ES cell therapy.
This work was funded by General program of Wuxi Hospital Management (Ygzxm14036).
The authors declare no conflict of interest.