2015 Volume 38 Issue 9 Pages 1430-1433
2015 Volume 38 Issue 9 Pages 1430-1433
A 56-year-old woman with systemic lupus erythematosus had bacteremia due to multidrug-resistant Pseudomonas aeruginosa (MDRP). She was initially treated with imipenem–cilastatin, tobramycin, and aztreonam; however, MDRP was still detected intermittently in her plasma. Multidrug-susceptibility tests demonstrated that MDRP was susceptible only to colistin. Therefore, in addition to these antibiotics, the administration of intravenous colistin methanesulfonate, a prodrug formula of colistin, was started at a daily dose of 2.5 mg/kg (as colistin base activity). The initial dose setting was based on the patient’s renal function (baseline creatinine clearance=32.7 mL/min). After initiating colistin, the patient’s C-reactive protein levels gradually decreased. Blood cultures showed no evidence of MDRP on days 8, 14, and 22 after colistin initiation. However, the patient’s renal function went from bad to worse owing to septic shock induced by methicillin-resistant Staphylococcus aureus (MRSA) infection. A few days later, the trough plasma levels of colistin were 7.88 mg/L, which appeared to be higher than expected. After decreasing the colistin dose, the patient’s renal function gradually improved. On the final day of colistin treatment, the plasma levels decreased to 0.60 mg/L. MDRP could not be detected in blood culture after colistin treatment. Therefore, we successfully treated a case of bloodstream infection due to MDRP by therapeutic drug monitoring (TDM) of colistin. It is suggested that the monitoring of blood colistin levels by liquid chromatography-tandem mass spectrometry can contribute to safer, more effective antimicrobial therapy of MDRP because TDM facilitates quick decisions on dose adjustments.
Colistin, known as an “old” antibiotic, has recently been reintroduced in clinical practice1–3) because of the increased emergence of infections caused by multidrug-resistant Gram-negative bacteria.2,4) Colistin was originally developed in Japan in the 1940s–1950s; however, initial studies reported adverse reactions, including nephrotoxicity or peripheral nervous system disorders.5) Because of these adverse events and the introduction of safer antibiotics, clinical use of colistin was abandoned in Japan in 1990.
On the other hand, some reports have indicated the usefulness of colistin against multidrug-resistant Pseudomonas aeruginosa (MDRP) infection.2,3)
Therapeutic drug monitoring (TDM) is a method used for determining the blood concentrations of drugs and optimizing the dose. Although a few case reports6,7) have been published on TDM of colistin, these data were retrospectively determined after the end of treatment. To the best of our knowledge, this is the first report on a case in which TDM-based dosing optimization of colistin was applied to treat an MDRP bloodstream infection.
As for the determination method of drug level, traditional determination systems using high-performance liquid chromatography (HPLC) require time-consuming sample pretreatment such as solid phase extraction and derivatization of colistin because of its poor UV absorption and no native fluorescence.8,9) For the detection of colistin level in blood, we utilized liquid chromatography-tandem mass spectrometry (LC-MS/MS), a widely used technology for determination of drug concentrations because of its high sensitivity and selectivity, which enabled us to monitor and maintain optimal blood colistin levels. The retention time of colistin on HPLC and LC-MS/MS is 15 min9) and 3 min, respectively. Furthermore, it requires relatively little time to detect colistin. Prompt monitoring to ensure appropriate plasma colistin levels will contribute to more favorable treatment because patients suffering from MDRP infections are at a risk of rapid deterioration and thus require early treatment. Here we report a case in which colistin dose was carefully adjusted using TDM.
Plasma colistin levels were determined using LC-MS/MS using the method reported by Jansson et al.10) with slight modification. In brief, 180 µL of plasma was spiked with 20 µL of colistin working solution (100, 50, 25, 12.5, and 0 mg/L), and polymyxin B was used as the internal standard. The samples were mixed with equal volumes of 0.1% trifluoroacetic acid in acetonitrile. After centrifugation at 15000 rpm for 15 min, the supernatant was mixed with an equal volume of 0.03% trifluoroacetic acid in water. Aliquots of the mixture (30 µL) were applied to LC-MS/MS.
EthicsThe present study was carried out in accordance with the guidelines for the care for human study, and the study protocol was approved by the ethics committee of the Hokkaido University Hospital.
A 56-year-old woman (body height, 150 cm and weight, 61.4 kg) with systemic lupus erythematosus had bacteremia due to MDRP. She had been treated with imipenem–cilastatin (IPM/CS; 500 mg, twice daily) against extended-spectrum beta-lactamase-producing Krebsiella pneumoniae, tobramycin (TOB; 2 mg/kg, every 48 h), which was the only sensitive antibiotic for initially detected Pseudomonas aeruginosa, and aztreonam (AZT; 1000 mg, twice daily) for 57 d; however, MDRP was still detected in blood intermittently. On the other hands, before detection in blood culture, MDRP was detected in a urine sample (62 d before the start of colistin treatment), and the patient presented with pyelonephritis due to the bacterial infection. Thus, the source of infection was thought to be from urinary tract in this case. Multidrug susceptibility tests using the breakpoint checkerboard plate method demonstrated that MDRP was susceptible only to colistin, whose minimum inhibitory concentration against MDRP was equal to or less than 1 mg/L (Fig. 1). Therefore, in addition to AZT, intravenous colistin (as a prodrug formula) was initiated at a daily dose of 2.5 mg/kg (as colistin base activity, Fig. 2). The initial dose setting of colistin was based on the guidelines of the Japanese Society of Chemotherapy for the appropriate use of colistin (released on July 2012). Prior to the administration of colistin, N-acetyl-β-D-glucosaminidase (NAG) levels in urine were 39.4 U/L and the urinary creatinine-adjusted value (NAG/Cre) was 158. Eight days after starting colistin, NAG and NAG/Cre levels were markedly elevated to 75.3 U/L and 290, respectively (Fig. 3). On the first day of colistin administration, serum creatinine levels and creatinine clearance (CCr, calculated using the Cockcroft–Gault equation) were 1.86 mg/dL and 32.7 mL/min, respectively. Until day 14 after initiating colistin, serum creatinine levels increased gradually to 2.77 mg/dL and CCr was 23.6 mL/min. On day 13, the trough levels of colistin were found to be higher (7.88 mg/L) than expected1) (Fig. 3). Therefore, the daily dosage of colistin was decreased to 1.5 mg/kg; however, the patient’s serum creatinine levels remained high for approximately 48 h, after which her renal function recovered, showing serum creatinine levels of 1.36 mg/dL on the day after decreasing colistin dosage (day 20, Fig. 2). On days 8, 14 and 22 after initiating colistin, MDRP could not be detected in her blood. Moreover, MDRP could not be detected in the blood culture even one week after the end of colistin treatment. Colistin was continued for 21 d and the C-reactive protein (CRP) levels markedly decreased from 13.75 to 2.47 mg/dL. Overall, the colistin treatment was clinically and bacteriologically effective. Meanwhile, 10 d after starting colistin, the patient fell into septic shock owing to methicillin-resistant Staphylococcus aureus (MRSA) infection. A subcutaneous abscess caused by MRSA under the sutured wound because of the aortic valve replacement after developing congestive heart failure was thought to be the source of MRSA sepsis. On this day, ceftazidime was started for the reinforcement of the treatment against the Gram-negative bacterial infection. After starting daptomycin (DAP), CRP and BT levels decreased markedly (Fig. 2). On the last day of colistin treatment, her BT and CRP levels had improved and her renal function recovered to nearly normal levels.

This assay is usually performed to evaluate the in vitro effects of the combination of multiple antibiotics against isolated MDRP in blood from this patient. Numbers mean concentration (mg/L) of each antibiotics which showed inhibitory effects against isolated strain. MEPM, meropenem; CAZ, ceftazidime; AZT, aztreonam; PIPC, piperacillin; AMK, amikacin; RFP, rifampicin; CPFX, ciprofloxacin; CL, colistin.
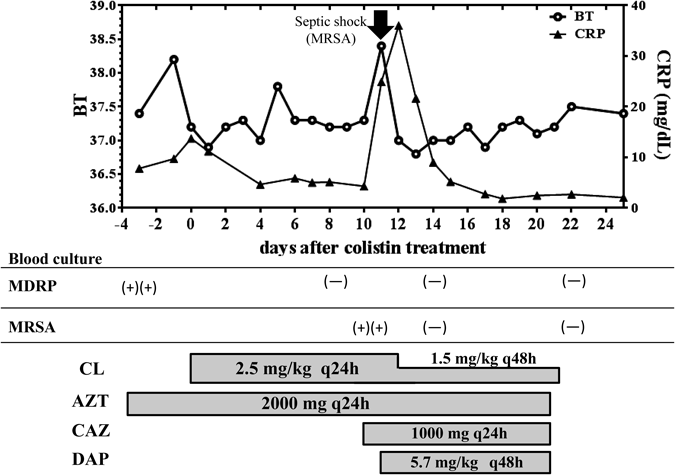
Colistin was initiated against blood-stream infection of MDRP. After starting colistin, MDRP in blood was disappeared on the day 8. Although this patient fell into septic shock evoked by MRSA infection (day 11, arrow), which was improved after starting daptomycin (DAP). BT, body temperature; CRP, C-reactive protein.

Renal function got worse after MRSA-evoked septic shock (day 11, arrow). NAG, N-acetyl-β-D-glucosaminidase; CRE, creatinine; s-cre, serum creatinine; CCr, creatinine clearance.
The emergence of MDRP is becoming one of the major clinical issues in nosocomial infections, particularly in critically ill patients. Colistin is considered the first line drug for MDRP but has not yet been approved in Japan. We therefore obtained a parenteral formula of colistin [as the prodrug colistin methanesulfonate (CMS)] from a pharmaceutical company outside Japan. Recently, Mizuyachi et al.11) reported the pharmacokinetic profiles of colistin and CMS in healthy Japanese male subjects, and Couet et al.12) reported the results in healthy Caucasian subjects. The plasma half-life (t1/2) of colistin is estimated to be approximately 3 h. However, in a study of critically ill patients, t1/2 of colistin was prolonged up to 14.4 h.1) In the previous study, a strong inverse trend was observed between steady-state plasma colistin levels and creatinine clearance. Thus, renal dosage and interval adjustment in colistin treatment are required, particularly for patients with kidney dysfunction.
A prospective observational cohort study demonstrated that trough plasma levels of colistin is an independent risk factor for nephrotoxicity13) and that trough plasma colistin levels of 3.33 mg/L on day 7 best predicted acute kidney injury (AKI). Information on the plasma levels of colistin will be helpful for future dosing recommendations. To promptly determine the colistin trough level, we utilized a new method based on LC-MS/MS without time-consuming preparation. We experienced trough colistin levels as high as 7.88 mg/L on day 13 after starting colistin treatment. The patient’s renal function tended to worsen, with creatinine clearance decreasing to 23.8 mL/min because of sepsis evoked by MRSA bloodstream infection; however, this transient renal dysfunction may have been partly due to colistin.
From day 14 after initiation of DAP for MRSA-induced sepsis, the patient’s renal function recovered gradually. On day 20 after starting colistin treatment, plasma colistin levels were decreased by 0.61 mg/L; this decline was thought to be due to the recovery of her creatinine clearance.
If the plasma levels of colistin are below the MIC value for a very large proportion of the dosage interval, there is a possibility that the treatment has failed.8) In the present case, MDRP in the blood culture was negative even one week after the end of colistin treatment. Her CRP level was remained lower than that on the day of starting colistin treatment, suggesting bacteriological and clinical effectiveness of colistin in this case. In addition, although the NAG/CRE value was examined only 2 points (Fig. 2), her daily urinary volume recovered to normal level (day 13 of colistin treatment, 150 mL, day 20, 2200 mL) after decreasing the colistin daily dose, suggesting that dose optimization of colistin is effective. These effects were due to TDM-based dosing of colistin, which contribute to avoid worsening of the renal function. Thus, TDM-based dosing of colistin is beneficial for patient safety. We were able to use colistin in a safer and more effective manner with TDM than without it. TDM-based colistin therapy will be a strong method for the patients suffering from MDRP.
The authors declare no conflict of interest.