2016 Volume 39 Issue 10 Pages 1723-1727
2016 Volume 39 Issue 10 Pages 1723-1727
An accumulating body of evidence suggests that males and females differ in vascular function in arteries under pathophysiological states. In this study, we tested whether there was a sex difference associated with serotonin (5-hydroxytryptamine, 5-HT)-mediated contraction in the carotid arteries of long-term streptozotocin (STZ)-induced diabetic rats [viz. 23 or 24 weeks after STZ (65 mg/kg, intravenously (i.v.)) injection starting at 8 weeks old of rats]. In the control group, the 5-HT- and high-K+-induced contractions were greater in females than in males. In both sexes, treatment with STZ led to a decrease of 5-HT-induced contraction in carotid arteries compared to controls. In STZ-induced diabetic rats, the carotid arterial 5-HT-induced contraction was greater in female rats than in diabetic male rats. The high-K+-induced contraction was greater in diabetic female rats than in either age-matched female controls or diabetic male rats. Expression of the 5-HT2A receptor, which is the main receptor for 5-HT-induced contraction in rat carotid arteries, was similar among the four groups. These results suggest that decreased 5-HT-induced carotid arterial contraction is seen in both sexes under long-term STZ-induced diabetic conditions. Further, this reduction seems to be weaker in females than in males. This alteration of 5-HT-induced contraction may be partly associated with increased voltage-dependent Ca2+ channel activity.
Although women generally develop cardiovascular diseases several years later than men, this benefit may be diminished in diabetic individuals. The results of numerous epidemiological studies investigating the relationship between sex and the development of cardiovascular disease in diabetics have been inconsistent.1,2) Alterations in the responsiveness of blood vessels to various hormones and/or neurotransmitters in patients of both sexes with diabetes mellitus are well established.3–6) However, there is confounding evidence of vascular function between diabetics of both male and females.
The neurotransmitter serotonin [5-hydroxytriptamine (5-HT)] is an important factor that is involved in the regulation of several vascular functions, including blood flow, blood pressure, and vascular tone, in pathophysiological states.7–9) Several reports by our research team10–12) and others3,13–15) have found that alterations in vasocontraction induced by 5-HT were observed in patients with cardiovascular diseases as well as in those with diabetes. An accumulating body of evidence suggests that 5-HT plays a role in the development of diabetic complications. For example, sarpogrelate, an antagonist of the 5-HT2A receptor, has beneficial effects against diabetic nephropathy, neuropathy, and diabetes-associated vascular dysfunction, including arterial stiffness and arteriosclerosis, in diabetic patients and animal models.16–19) This relevant evidence suggests that the manipulation of 5-HT function can represent a critical therapeutic target in the case of diabetic vasculopathies. In the carotid artery, which supplies blood to the brain, we found increased 5-HT-induced contractions in type 2 diabetic Goto–Kakizaki rats.11) Moreover, we very recently demonstrated that exposure to high insulin levels, but not high glucose levels, can increase 5-HT-induced contraction in rat carotid arteries.20) However, at present, whether there are sex-associated differences in carotid arterial contraction induced by 5-HT in chronic-stage diabetes remains unclear.
Type 1 diabetes can be induced by the injection of streptozotocin (STZ), which causes β-cell death in the pancreas and is commonly used to induce type 1 diabetes in experimental models.21) The aims of our study were to investigate whether 5-HT-induced carotid arterial contraction would differ between males and females with STZ-induced diabetes.
Male and female Wistar rats (8 weeks old) were randomly divided into diabetic and non-diabetic (control) groups. Experimental type 1 diabetic rats were induced with a single intravenous injection of STZ (65 mg/kg dissolved in citrate buffer), as described previously.22–24) As a control, age-matched rats were injected with citrate buffer alone. All animals were given a standard laboratory diet and water ad libitum until the rats were 31 or 32 weeks old (viz. 23 or 24 weeks after STZ/buffer injection). This study was approved by the Hoshi University Animal Care and Use Committee, and all experiments were performed in accordance with “Guide for the Care and Use of Laboratory Animals” published by the U.S. National Institutes of Health and “Guide for the Care and Use of Laboratory Animals” adopted by the Committee on the Care and Use of Laboratory Animals of Hoshi University.
Measurement of Blood Parameters and Blood PressurePlasma (nonfasting; taken at sacrifice) parameters and blood pressure (measured at 1 week before sacrifice) were measured as reported previously.25–27) Blood glucose was measured by a glucose meter (OneTouch Ultra, LifeScan, Johnson & Johnson Company, Milpitas, CA, U.S.A.). Plasma lipid parameters and plasma insulin were measured using commercially available kits (Wako Pure Chemical Industries, Ltd., Osaka, Japan and Shibayagi, Gunma, Japan, respectively). Systolic blood pressure was measured by the tail-cuff technique (model BP-98A; Softron, Tokyo, Japan).
Functional StudyVascular isometric force of the common carotid artery was recorded as described previously.20,28,29) To generate concentration–response curves in modified Krebs–Henseleit solution [(in mM) 118.0 NaCl, 4.7 KCl, 25.0 NaHCO3, 1.8 CaCl2, 1.2 NaH2PO4, 1.2 MgSO4, and 11.0 glucose] and 5-HT (serotonin hydrochloride, Sigma-Aldrich, St. Louis, MO, U.S.A) (10−9–3×10−5 M) or high-K+ (10–80 mM) was added cumulatively to the bath until a maximal response was achieved. To assess concentration–response curves for high-K+ (10–80 mM), we removed an appropriate volume of solution from the bath and then added equal volumes of high [K+] solution as reported previously.11,30)
ImmunoblottingWestern blotting and data analysis were performed as previously described.11,20,26) Primary antibodies were used as follows: 5-HT2A receptor (1 : 500, ImmunoStar, Hudson, WI, U.S.A) and β-actin (1 : 5000, Sigma-Aldrich).
Data and Statistical AnalysisData were expressed as the mean±standard error (S.E.). Concentration–response curves were analyzed using a nonlinear regression curve-fitting program (Graph Pad Prism software ver. 5.0 for Mac, San Diego, CA, U.S.A.). Statistical analysis was performed using a one-way ANOVA followed by a Bonferroni’s test for multiple comparisons. Statistical analysis of concentration–response curves were performed using a two-way ANOVA with repeated measures followed by a Bonferroni post-hoc test. * p Values <0.05 were considered significant.
As shown in Table 1, treatment with STZ (vs. control group) led to significant increases in left ventricle/body weight (BW) ratios, as well as glucose levels; however, significant decreases in BW and heart rate were observed in both the male and female groups. In male rats, STZ led to a significant increase blood pressure and a decrease in insulin levels and tended to increase total cholesterol and triglyceride levels. In female rats, STZ led to a significant increase in total cholesterol and triglyceride levels and tended to increase blood pressure. Surprisingly, the insulin levels were similar between the control and STZ groups in female rats.
| Male | Female | |||
|---|---|---|---|---|
| Control | Diabetic | Control | Diabetic | |
| Body weight (g) | 613.2±9.4 (10) | 368.6±14.2 (8)* | 332.7±6.1 (9)* | 245.5±9.3 (9)#† |
| LV/BW (mg/g) | 1.62±0.03 (10) | 2.50±0.07 (8)* | 1.94±0.05 (9)* | 2.54±0.11 (9)# |
| SBP (mmHg) | 105±4 (10) | 136±6 (8)* | 103±3 (9) | 120±4 (9) |
| HR (beats/min) | 332.1±10.2 (10) | 254.3±9.4 (8)* | 354.4±16.5 (9) | 253.9±9.3 (9)# |
| Glucose (mg/dL) | 111.8±3.3 (10) | 550.8±15.6 (8)* | 119.0±4.5 (9) | 581.0±10.9 (9)# |
| Insulin (ng/mL) | 1.82±0.3 (8) | 0.79±0.08 (8)* | 1.51±0.08 (7) | 1.36±0.10 (7) |
| T-Chol (mg/dL) | 115.8±6.5 (10) | 145.6±6.9 (7) | 70.4±6.7 (9)* | 137.1±13.2 (9)# |
| TG (mg/dL) | 205.6±23.1 (10) | 417.8±47.6 (7) | 215.0±21.1 (9) | 461.4±90.7 (9)# |
| Ring weight (mg) | 0.93±0.05 (8) | 0.85±0.07 (8) | 0.62±0.04 (9)* | 0.51±0.04 (9)† |
Values are the mean±S.E. Number of experiments is shown within parentheses. LV; left ventricule, BW; body weight, SBP; systolic blood pressure, HR; heart rate, T-Chol; total cholesterol, TG; triglyceride. * p<0.05 vs. male control, # p<0.05 vs. female control, † p<0.05 vs. male diabetic.
The ring weight of the carotid artery was different between male and female rats with and without diabetes, whereas diabetes did not differentially affect the weight in either sex (Table 1). As shown in Fig. 1, diabetes led to decrease 5-HT-induced contraction in both sexes; whereas, high-K+-induced contraction did not change in males but increased in female rats (viz. control vs. diabetics). When we focused on sexes, both contractions induced by 5-HT and high-K+ increased in females (vs. males) with and without diabetes.
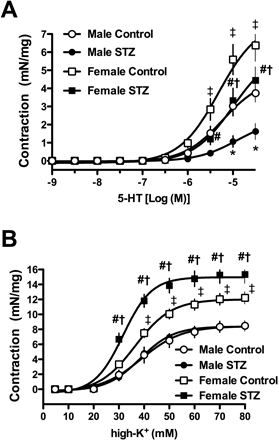
The y-axis shows the increase in tension (mN/mg) measured at the peak response. Data are expressed as the mean±S.E. n=8 or 9. * p<0.05, male control vs. male STZ. # p<0.05, female control vs. female STZ. † p<0.05, male STZ vs. female STZ. ‡ p<0.05, male control vs. female control.
To investigate the possible mechanisms underlying the sex-associated differences associated with 5-HT-mediated contraction in STZ-induced rats, we examined protein expression of the 5-HT2A receptor.7) Unexpectedly, the expression was similar among the four groups (Fig. 2).
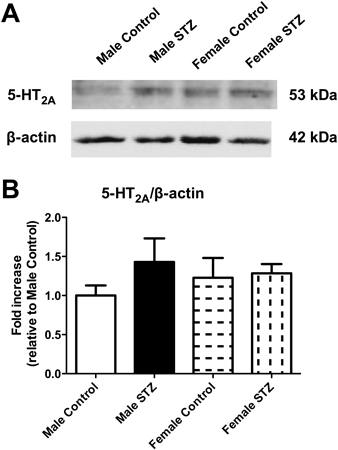
(A) Representative Western blot is presented. (B) Corresponding densitometric analysis showing the expression of the 5-HT2A receptor and β-actin. Results are presented as the fold increase relative to male control. Data are expressed as the mean±S.E. n=5 or 6.
There is confounding evidence in the literature concerning the alteration of 5-HT-induced contraction in the arterial vessels of diabetes. In this study, long-term diabetes induced by STZ led to a reduction of 5-HT-induced contraction in rat carotid arteries in both male and female rats. Notably, the extent of the reduction of 5-HT-induced contraction was lower in females than in males: the contractile response to 5-HT was greater in the female diabetic group than in the male diabetic group. Nuno and Lamping3) found that 5-HT-induced contraction was greater in mouse aortas of nondiabetic males than in those of females and that following diabetes development, 5-HT-induced contraction increased in males but not in females. Their study used diabetic mice for functional analysis at 12–16 weeks after the first measurement of hyperglycemia and injected STZ (2–3 injections at 100–150 mg/kg, intraperitoneally (i.p.)). Sheykhzade et al.31) found that 5-HT-induced contraction in coronary arteries was similar between age-matched female control rats and long-term (34 weeks) STZ (65 mg/kg, intravenously (i.v.) at 10 weeks old)-induced diabetic female Wistar rats. Van Buren et al.32) observed that in mesenteric resistance arteries in short-term (4 weeks) and long-term (40 weeks) STZ (40 mg/kg, i.v.)-induced diabetic rats, the 5-HT-induced contraction was similar to age-matched control rats; however, the 5-HT-induced contraction increased and decreased in the basilar artery in short-term and long-term STZ-induced diabetic rats, respectively. These discrepancies may result from vessel types, sex, or duration of disease.
The STZ-induced diabetic method is commonly used to generate type 1 diabetic animal models.21,33) In this study, male rats treated with a single dose of STZ exhibited lower BW, hyperglycemia, and hypoinsulinemia than controls. However, in female rats (vs. controls), hypoinsulinemia was not seen in STZ-injected rats despite the expression of hyperglycemia and lower BW. These paradoxical data may be a result of β-cell recovery in female rats over time. Indeed, several reports suggested that STZ-injected female rats showed signs of partial spontaneous regeneration with increased circulating and pancreatic insulin levels within 1 year of the disease duration.34,35) Such partial recovery from STZ-induced diabetes has been also found in adult rats treated with low concentration of STZ.36) The lower susceptibility to the diabetogenic effects of STZ has been observed in female37) and castrated male mice.38) These findings suggest that STZ sensitivity and/or status of β cells may be different between males and females; however, further investigations on these findings are required. In the present study, we also found increased levels of blood lipid parameters (namely, total cholesterol and triglyceride) in the diabetic group compared with those in the control group in each sex, and the alteration of reactivity to 5-HT was seen in hyperlipidemic conditions.39,40) Because the above blood parameters did not differ between male and female diabetic rats, these factors may not be associated with 5-HT-induced contraction in carotid arteries. Moreover, we very recently found that high insulin exposure, but not high glucose levels, led to enhanced 5-HT-induced contraction in rat carotid arteries.20) Therefore, we suggest that the insulin level is a determinant factor in contractile response to 5-HT in carotid arteries. Further investigation is required to identify causative factors in the alteration of 5-HT-induced vasocontraction between sexes.
Vascular contractile mechanisms are mainly divided into two mechanisms: calcium signals and changes in the contractile apparatus’ sensitivity to calcium.41,42) Indeed, these two signaling pathways are utilized in 5-HT2A receptor-mediated responses in vascular smooth muscle.9,11–14,20,43) In the present study, high-K+-induced contraction in diabetic female rats was greater than in control female rats and diabetic male rats. We suggest that the greater response to 5-HT in diabetic female carotid arteries relative to that of diabetic males may be partly attributable to increased voltage-gated calcium channel activity rather than 5-HT2A receptor expression. Because insulin can modulate intracellular calcium levels,44,45) the different insulin levels under diabetic conditions between sexes may be also contributed to altered high-K+-induced contraction. However, further investigation is required to establish the mechanisms underlying these factors.
In conclusion, we suggest that decreased 5-HT-induced carotid arterial contraction is observed in both sexes under long-term STZ-induced diabetes and the extent of the reduction differs between sexes. Our findings provide evidence of sex differences related to reactivity to 5-HT in long-term diabetic states and may contribute to the development of new strategies for the treatment of diabetes-associated vascular dysfunction.
We thank H. Sashikubi, A. Suwa, M. Takeuchi, M. Takahashi, M. Nagata, S. Hotozuka, J. Nomoto, and M. Majima for technical assistance. This study was supported in part by JSPS KAKENHI Grants 26460107 (to T.M.), 15K21419 (to K.T.), and 15K07975 (to T.K.).
The authors declare no conflict of interest.