2016 Volume 39 Issue 11 Pages 1881-1887
2016 Volume 39 Issue 11 Pages 1881-1887
Disulfiram (DSF) is a dimer of diethyldithiocarbamate (DDC) that we previously added to a solution of 2-hydroxypropyl-β-cyclodextrin (DSF solution). We found that the instillation of this DSF solution delayed lens opacification in a hereditary cataractous ICR/f rat. In this study, we attempted to design an ophthalmic formulation containing DSF nanoparticles for use as a lens targeted drug delivery system (nano-DSF suspension), and investigated the changes in drug content in the lens after the instillation of DSF solution or nano-DSF suspension. The nano-DSF suspension was prepared by a bead mill method to yield a mean particle size of nano-DSF of 181 nm. Following the instillation of 1.4% DSF solution or the nano-DSF suspension, DDC was detected only in the aqueous humor and lens; in both, the area under the curve (AUC) and mean residence time (MRT) for the nano-DSF suspension were higher than for the DSF solution. In addition, we found that the DDC residence time in the cortex and nucleus of the lens was higher than in the capsule-epithelium. Although DDC was not detected in the cortex and nucleus of lenses following the instillation of the 1.4% DSF solution, the instillation of a 1.4% nano-DSF suspension led to the accumulation of DDC in both areas. In conclusion, it is possible that the instillation of a nano-DSF suspension can supply more DDC into the aqueous humor and lens than a conventional formulation, and these findings provide information significant for the prevention of cataracts and the design of a lens targeted drug delivery system.
Cataracts are the leading cause of treatable blindness worldwide, and cataract surgery and intraocular drugs have been used as therapy. However, the availability of cataract surgery and intraocular drugs is not sufficient in developing countries, and WHO has reported cataracts as the main cause of low vision in the many developing countries.1) Based on this background, the design and improvement of anti-cataract eye drops is very important.
Eye drops comprise the major ophthalmic preparation, and their use is both convenient and safe. However, a high level of transcorneal penetration following the instillation of eye drops is necessary for drug delivery into the lens. Furthermore, the concentration of drugs administered as eye drops is diluted to approximately 20% by lacrimal fluids.2) In addition, the drops are lost from the tear film within 0.5–2 min, with eye drop components excreted through the nasolacrimal duct into the mouth,2) with a small amount remaining associated with the conjunctival tissue.2) Therefore, with traditional eye drops, only small amounts of the administered drug actually penetrate the cornea to reach the desired lens tissue due to corneal barriers and dilution caused by lacrimal fluids.3–5) In an attempt to overcome these problems, several strategies have been devised using microparticles, viscous solutions and hydrogels.3,6–10) Recently, it has been reported that drug penetration across the cornea can be significantly improved by decreasing the particle size of the drug using nanoparticles.5,9,11) We have prepared solid nanoparticles of such drugs as indomethacin, tranilast, cilostazol, and disulfiram (DSF), and reported that the abilities of these drugs to penetrate across the cornea can be significantly improved using solid nanoparticles.12–16) It is expected that ophthalmic drug delivery systems using nanoparticles may provide sufficient amounts of drugs into the lens.
DSF is a dimer of diethyldithiocarbamate (DDC). We preciously prepared a 2-hydroxypropyl-β-cyclodextrin (HPβCD) solution containing DSF (DSF solution), and found that its instillation delayed lens opacification in a recessive-type hereditary cataractous strain of rats (ICR/f and UPL rats).17–20) In addition, we reported that the induction of inducible nitric oxide synthase (iNOS) via nuclear factor-kappa B (NF-κB) produces excessive nitric oxide (NO), and that this excessive NO causes enhanced lipid peroxidation and a decrease in cytochrome c oxidase in the lenses of hereditary cataractous strains of rats during cataract development.18,19) The enhanced lipid peroxidation and decrease in cytochrome c oxidase lead to a decrease in Ca2+-ATPase function, resulting in lens opacification via the enhancement of Ca2+ content in the lens.18,19) The ophthalmic formulation containing DSF has the ability to attenuate the expression of iNOS, resulting in a delay in cataract development.17–20) Furthermore, we designed a high quality suspension containing DSF nanoparticles (nano-DSF suspension), and showed that the corneal penetration and corneal residence time of DSF from this nano-DSF suspension were significantly higher than from a regular DSF solution (conventional eye drops).15) In this study, we investigate the changes in drug contents in the lenses of rats instilled with the DSF solution and nano-DSF suspension.
Seven-week-old Wistar rats (male) were obtained from Kiwa Laboratory Animals Co., Ltd. (Wakayama, Japan), and used in this study. The experiments were performed in accordance with the Kindai University (formerly Kinki University) Faculty of Pharmacy Committee for the Care and Use of Laboratory Animals and the Association for Research in Vision and Ophthalmology resolution on the use of animals in research.
Preparation of DSF Solution and Nano-DSF SuspensionDSF powder (0.5 or 1.4%) and 0.5% methylcellulose (MC), 0.1% D-mannitol, 0.001% benzalkonium chloride (BAC) were added to HPβCD (5 or 12%) in saline, and the mixtures were stirred for 24 h in the dark (room temperature). Solutions containing DSF were filtered through a 0.20 µm membrane filter (DSF solution).20) Suspensions containing DSF nanoparticles (nano-DSF suspension, pH 6.5) were prepared by a beads mill method according to a previous report.15) DSF powder (0.5 or 1.4%), 0.5% MC, 0.1% D-mannitol, and 0.001% BAC were mixed with Zirconia beads (diameter: 2 mm), and crushed with a Bead Smash 12 (Wakenyaku Co., Ltd., Kyoto, Japan) for 30 s (3000 rpm, 4°C). The mixtures were then mixed with 0.1 mm zirconia beads in saline (5500 rpm, 30 s×15 times, 4°C), and crushed again with the Bead Smash 12. The particle size of the nano-DSF suspension determined using a SALD-7100 (Shimadzu Corp., Kyoto, Japan) was 181±90 nm (the mean±standard deviation (S.D.), refractive index 2.30–0.05i).
Instillation of DSF Solution and Nano-DSF SuspensionThe DSF solution or nano-DSF suspension was instilled into the eyes of rats (30 µL) with the eyes held open for about 1 min to prevent the DSF solution or nano-DSF suspension from being washed out. In the case of repetitive instillations, the 30 µL of DSF solution or nano-DSF suspension were instilled into the eye three times a day (9:00 a.m., 1:00 p.m. and 7:00 p.m.) for 1, 7, 14 or 21 d, and the lens (total lens) was removed 12 h after the final instillation.
Measurement of DSF and DDC ConcentrationsDSF and DDC concentrations were determined by the following HPLC method according to previous reports.15,20) Three micrograms of benzophenone was added to the samples as an internal standard, and the samples were filtered through a 0.45 µm Chromatodisk 4A (Kurabo Industries Ltd., Osaka, Japan). The drug concentrations were measured on a Shimadzu LC-20AT system equipped with a column oven CTO-20 A (Shimadzu Corp.) and an Inertsil® ODS-3 (3 µm, column size: 2.1×50 mm) column. The HPLC conditions were as follows: mobile phase, 45% acetonitrile containing 0.1% trifluoroacetic acid; flow rate, 0.25 mL/min; wavelength for detection, 215 nm; column temperature, 35°C.
DSF and DDC Contents in the Aqueous Humor and LensWistar rats were euthanized by injection of a lethal dose of pentobarbital sodium 0–40 min after the instillation of DSF solution or nano-DSF suspension (30 µL) the eyes, and the aqueous humor (5 µL) and lenses were removed. The lenses were homogenized in 50 µL saline, and the homogenates were centrifuged at 15000 rpm, 20 min, 4°C. The supernatants from lens homogenates and aqueous humor samples were used for the measurement of DSF and DDC concentrations. The DSF and DDC concentrations in the aqueous humor (CAH) and lens (CLens) were determined by HPLC as described above. The area under the curve of the DDC concentration versus time (AUCAH or Lens), the area under the first moment curve (AUMCAH or Lens), and the mean residence time (MRTAH or Lens) in the aqueous humor or lens were calculated according to the following equations (Eqs. 1–3):
 | (1) |
 | (2) |
 | (3) |
The epithelium containing capsules (capsule-epithelium) was carefully removed and separated from the nucleus and cortical portions. The cortex was also separated, and the remaining lens other than the capsule-epithelium and cortex was used as the nucleus.
In Vitro DDC Absorption into LensWistar rats were euthanized by injection of a lethal dose of pentobarbital sodium, and the lenses were removed. The isolated lenses were treated with 80 or 160 µM DDC for 0–40 min. After that, the lenses were gently washed in saline, and the lens (total lens) was divided to the capsule-epithelium, cortex and nucleus as described above. The samples were homogenized in 50 µL saline, and the homogenates were centrifuged (15000 rpm, 20 min, 4°C). The supernatants were used for the measurement of DDC concentrations. The DDC concentrations were determined by HPLC as described above.
In Vitro DDC Residence in LensWistar rats were euthanized by injection of a lethal dose of pentobarbital sodium, and the lenses were removed. The isolated lenses were treated with 80 or 160 µM DDC for 40 min. Then, the lenses were gently washed in saline, and incubated in saline for 0–40 min. The incubated lenses (total lens) were divided into the capsule-epithelium, cortex and nucleus as described above, homogenized in 50 µL saline, and centrifuged (15000 rpm, 20 min, 4°C). The DDC concentrations in the supernatants were measured by HPLC as described above.
Statistical AnalysesThe data were by the Unpaired Student’s or Aspin–Welch’s t-tests and ANOVA followed by Dunnett’s multiple comparison; p Values less than 0.05 were considered significant.
We previously prepared the 0.5% DSF solution by using HPβCD (DSF solution) and reported that the instillation of 0.5% DSF solution delayed the cataract development in the cataract model.17) On the other hand, we also showed that a development of the DSF eye drops containing higher than 0.5% was important for strongly inhibition of the lens opacification. However, high concentration of 2-hydroxypropyl-β-cyclodextrin need to prepare the solution containing high DSF amount (12% HPβCD need to prepare the 1.4% DSF solution), and the high concentration of HPβCD cause the irritation to cornea.21) Recently, we designed the ophthalmic formulation containing drug nanoparticles, and the corneal toxicity of ophthalmic formulation was lower than that in conventional formulation containing 2-hydroxypropyl-β-cyclodextrin.15) From these previous results, we determined the DSF concentration (0.5, 1.4%), and investigated the supply of drug into lens by the instillation of DSF eye drops in this study. Figure 1 shows the changes in DDC contents of the aqueous humor and lenses of rats instilled with DSF solutions (A, C) or nano-DSF suspension (B, D). Table 1 shows the pharmacokinetic parameters calculated from the data in Fig. 1.
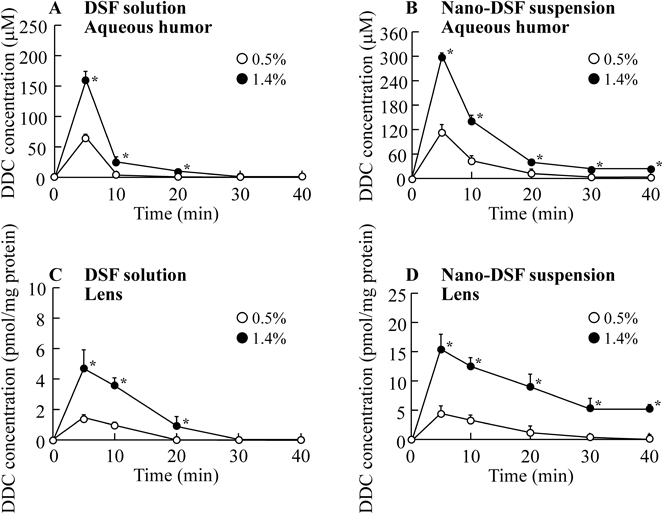
No DSF was detected in the aqueous humor or lens. Open circles, 0.5% DSF-instilled rats; closed circles, 1.4% DSF-instilled rats. The data are presented as the mean±S.E. of 4–8 independent rats. * p<0.05, vs. 0.5% DSF-instilled rats for each category.
| Aqueous humor | Lens | |||
|---|---|---|---|---|
| AUCAH (µM·min) | MRTAH (min) | AUCLens (pmol/mg protein·min) | MRTLens (min) | |
| 0.5% DSF solution | 3.97×102 | 9.14 | 1.51×10 | 9.70 |
| 1.4% DSF solution | 1.10×103 | 9.43 | 5.89×10 | 1.05×10 |
| 0.5% Nano-DSF suspension | 1.19×103 | 1.18×10 | 6.05×10 | 1.23×10 |
| 1.4% Nano-DSF suspension | 3.26×103 | 1.21×10 | 3.37×102 | 1.75×10 |
Data (mean values) shown in Fig. 1 were used for the estimation of these parameters, calculated according to Eqs. 1–3.
DSF was not detected (only dissolved DDC was detected) in the aqueous humor or lens of rats instilled with either the DSF solutions or nano-DSF suspension. Peak of DDC levels were observed 5 min after the instillation of either the DSF solutions or nano-DSF suspension. In the case of the DSF solutions, the DDC levels in the aqueous humor decreased rapidly over 5–10 min; DDC levels fell below the detection level at 20 min after instillation in the case of the 0.5% and 30 min after instillation in the case of the 1.4% DSF solution (Fig. 1A). The AUCAH value for the 1.4% DSF solution-instilled rats was approximately 2.8-fold higher than that for the 0.5% DSF solution-instilled rats; the MRTAH values were similar for both concentrations (Table 1). On the other hand, the DDC levels in aqueous humor of rats instilled with the nano-DSF suspension were higher than found for the DSF solutions, and the MRTAH value was also higher (Table 1, Fig. 1B). We previously investigated the transcorneal penetration of ophthalmic formulations containing DSF micro or nanoparticles using rabbit corneas, and found that DSF nanoparticles in ophthalmic formulations attach to the cornea and conjunctiva, and the dissolved DSF passes through the cornea.15) Moreover, the dissolved DSF after the instillation of ophthalmic formulations is converted to dissolved DDC via catalysis by a sulfhydryl (SH)-residue-containing protein (ALDH3A1) in the cornea.22) We also reported that the amount of DSF and its residence time in the cornea after the instillation of the nano-DSF suspension were enhanced in comparison with DSF solutions in in vivo experiments using rabbits.15) Based on these previous reports and the data in this study, we suggest that the mechanism of DDC transcorneal penetration is similar in both rabbit and rat.
Furthermore, we measured changes in the DDC levels in the lenses of rats instilled with the DSF solutions or nano-DSF suspension. Only dissolved DDC was detected in the lens, with the peak DDC level observed at 5 min after instillation for all tested formulations (Figs. 1C, D). The DDC levels in the lens were lower than in the aqueous humor, while no DDC was detected in the blood after the DSF instillation (data not shown). These results suggest that DDC in the lens is supplied from the aqueous humor. In contrast to the results in aqueous humor, the MRTLens values were prolonged with increasing DSF concentration in the ophthalmic formulations, and the MRTLens value for the nano-DSF suspension was higher in comparison with the DSF solutions. Therefore, we have demonstrated DDC absorption (Fig. 2) and residence (Fig. 3) in lenses treated in vitro with DSF solutions in order to elucidate the differences in the MRT of DDC in the aqueous humor and lens.

Isolated lenses were treated with 80 or 160 µM DDC for 0–40 min. The DDC-treated lenses (total lens) were divided into the capsule-epithelium, cortex and nucleus, and the DDC levels were measured in total lens (A), capsule-epithelium (B), cortex (C) and nucleus (D). Open circles, rat lenses treated with 80 µM DDC; closed circles, rat lenses treated with 160 µM DDC. The data are presented as the mean±S.E. of 5–6 independent rat lenses.

Isolated lenses were treated with 80 or 160 µM DDC for 40 min. After that, the lenses were incubated in saline for 0 min (control) or 5–40 min. The incubated lenses (total lens) were divided into the capsule-epithelium, cortex and nucleus, and DDC levels were measured in total lens (A), capsule-epithelium (B), cortex (C) and nucleus (D). Open circles, rat lenses treated with 80 µM DDC; closed circles, rat lenses treated with 160 µM DDC. The data are presented as the mean±S.E. of 5–6 independent rat lenses. * p<0.05, vs. control (0 min) for each category.
The DDC concentrations in total lens treated with 80 or 160 µM DDC for 40 min were approximately 41.6 or 83.5 pmol/mg protein, respectively. Although DDC absorption into the capsule-epithelium reached a plateau approximately 5 min after treatment, little DDC absorption into the cortex and nucleus were observed until 20 min after treatment (Fig. 2). DDC residence time in the capsule-epithelium was shorter than in the cortex or nucleus, with approximately 73% (80 µM) or 80% (160 µM) of the DDC in capsule-epithelium being released within 10 min after the start of the experiment (Fig. 3B). In addition, no DDC release from the cortex or nucleus was observed for 40 min after the start of the experiment (Figs. 3C, D). These results show that DDC residence in lens is related to the difference between the DDC residence time in the aqueous humor and lens, and that DDC accumulation in the cortex and nucleus enhance the MRTLens. Prolonging the drug exposure time may be an effective way to increase the amount of DDC permeating into the cortex and nucleus since the accumulation of DDC levels in the cortex and nucleus of lenses treated with DDC was only observed after 20 min from the start of the experiment (Figs. 2C, D). On the other hand, it is important to elucidate the precise mechanism for the regulation of DDC in lens. The lens, which is composed of epithelial cells on the anterior surface of the lens and fiber cells, grows with an increase in age, and the epithelial cells move towards the lens nucleus portions during the growth. In this process, the epithelial cells were developed to become fiber cells. Differentiation into a lens fiber cell is accompanied by changes in cell shape, the expression of crystallins and the degradation of cellular organelles (the cells in cortex and nucleus is fiber cell, and have no nuclei and organelles). From those organizational structure, the capsule-epithelium works as control membrane for the drug absorption and release in lens, since the cell density in capsule-epithelium is higher than that in cortex and nucleus. Therefore, the drug absorption and residence may be regulated by the capsule-epithelium. In addition, it was suggested that the DDC amount in capsule-epithelium was release from lens for 0–10 min, and the DDC diffused from cortex and nucleus was determined in capsule-epithelium for 10–40 min. Moreover, the DDC decrease in capsule-epithelium for 0–10 min may affect the changes in total lens for 0–10 min (Fig. 3).
The MRTAH for the nano-DSF suspension was higher than that for the conventional eye drops (solution type) in this study. Therefore, the prolonging the drug exposure time may be able to supply DDC into the cortex and nucleus. Based on this hypothesis, we measured the DDC concentration in the lens 12 h after repetitive instillation (three times a day) of DSF solution or nano-DSF suspension (Fig. 4). No DDC was detected in the lens (capsule-epithelium, cortex or nucleus) following the repetitive instillation of DSF solution. However, the repetitive instillation of the nano-DSF suspension enhanced the DDC content in the capsule-epithelium, cortex and nucleus. Although Fig. 2B suggested the highest absorption of DDC in capsule-epithelium, the lowest DDC concentration was exhibited by capsule-epithelium (Fig. 4). In the Fig. 4 (in vivo study), the lens was removed 12 h after the final instillation. Therefore, the DDC amount in capsule-epithelium may be released into the aqueous humor, since Fig. 3B showed that the DDC in capsule-epithelium being released within 10 min after the start of the experiment, and the DDC diffused from cortex and nucleus was determined in capsule-epithelium (Fig. 4). In addition, the DDC content in the cortex and nucleus also increased with the length of the instillation period. We showed that DDC accumulation in the cortex and nucleus enhanced the MRTLens (Fig. 3, Table 1), and the DDC amount was detected 12 h after the instillation of nano-DSF suspension (Fig. 4). These results suggested that the DDC content in the cortex and nucleus was accumulated by the repetitive instillation of nano-DSF suspension. These results support the finding that DDC residence in the cortex and nucleus is longer than in the capsule-epithelium, and indicates that an ophthalmic formulation containing nanoparticles may be useful as a lens targeted drug delivery system.

One-point-four percent DSF solution or the nano-DSF suspension was instilled into the eyes of rats three times a day (9:00 a.m., 1:00 p.m. and 7:00 p.m.) for 1, 7, 14 or 21 d. The lens (total lens) was removed 12 h after the final instillation, and divided into the capsule-epithelium, cortex and nucleus. DDC levels were measured in the total lens (A), capsule-epithelium (B), cortex (C) and nucleus (D). No DSF was detected in the lenses of rats instilled with the DSF solution or nano-DSF suspension. Open circles, 1.4% DSF solution-instilled rat; closed circles, 1.4% nano-DSF suspension-instilled rat. The data are presented as the mean±S.E. of 4–8 independent rat lenses. N.D., not detected. * p<0.05, vs. lenses of rats instilled with nano-DSF suspension for 1 d.
Further studies are needed to improve the lens targeted drug delivery system using nanoparticles. In addition, it is important to clarify the effect of ophthalmic nano-DSF suspensions on cataracts. Therefore, we will investigate the therapeutic effects of an ophthalmic nano-DSF suspension on lens opacification using a hereditary cataractous strain (ICR/f rat).
In conclusion, we have designed an ophthalmic formulation containing DSF nanoparticles. The instillation of the nano-DSF suspension supplied more DDC into the lens than a conventional formulation (DSF solution), and repetitive instillation of the nano-DSF suspension enabled DDC delivery and accumulation into the cortex and nucleus of the lens. In addition, we have shown that the DDC residence time in the cortex and nucleus of lenses is higher than in the capsule-epithelium. Furthermore, we have demonstrated drug behavior in the lens after the instillation of eye drops in terms of three factors: (I) peak drug concentration in the aqueous humor, (II) drug residence time in the aqueous humor, and (III) drug residence time in the lens. These findings provide information significant for the search for therapies to prevent cataracts and the design of lens targeted drug delivery systems.
The authors declare no conflict of interest.