2016 Volume 39 Issue 12 Pages 1932-1938
2016 Volume 39 Issue 12 Pages 1932-1938
Stroke-prone spontaneously hypertensive rats (SHRSP/Izm; SHRSP) develop severe hypertension and die of cerebral stroke. However, the genetic mechanisms underlying their stroke susceptibility have not been clarified yet. In this study, we used astrocytes from the newborn brain cortex of spontaneously hypertensive rats (SHR/Izm; SHR) and SHRSP to find the difference of genetic characteristics. Astrocytes are known to have functions of vasodilation and nutrient uptake for neurons in the brain. The continuous generation of hydrogen peroxide (H2O2) dose-dependently causes cell death in astrocytes, and SHRSP was more vulnerable than SHR. We found that the total thiols decreased in SHRSP astrocytes but the total glutathione (GSH) did not change. Hydrogen sulfide (H2S), which is known to protect cells through anti-oxidant and vasodilatory effects, is produced by cystathionine β-synthase (CBS) in astrocytes. We found that H2S production was significantly decreased in SHRSP as compared to SHR. This was caused by the decreasing expression of mRNA, protein and enzyme activity of CBS in astrocytes. We also found that astrocyte cell death from oxidative stress could be prevented by GYY4137 H2S donor. H2S is also known to cause protein S-sulfhydration to modify enzyme activity. Sulfane sulfur in astrocytes was significantly lower in SHRSP and decreased by CBS inhibitor. We showed that astrocytes in SHRSP vulnerable to oxidative stress may be caused by reduction of H2S through lower expression and activity of CBS.
Stroke-prone spontaneously hypertensive rat (SHRSP/Izm; SHRSP) was produced by selective mating from spontaneously hypertensive rat (SHR/Izm; SHR). SHRSP develops severer hypertension than SHR, with a more than 95% death rate due to cerebral stroke.1)
Long-term hypertension causes reduction of cerebral blood flow in SHRSP.2,3) It causes hypoxia and produces reactive oxygen species (ROS). Recently, Morikawa et al. reported that a hypoxic condition induces vasodilatation through the production of hydrogen sulfide (H2S) in the brain.4) H2S, a well-known cytotoxic gas, has recently been shown to be an endogenously produced signaling molecule. It is a gasotransmitter, like nitric monoxide (NO) and carbon monoxide (CO). Some studies suggest that H2S has cytoprotection through anti-oxidant effect and vasodilatation via ATP-dependent potassium channel opening.5–8) H2S is produced from cysteine and homocysteine by cystathionine β-synthase (CBS) in brain. CBS has been found in regions of the brain particularly localized in astrocytes.9)
H2S also modulates the activity of enzymes through S-sulfhydration at cysteine residues.10) Recently, molecular species containing sulfane sulfur, such as persulfide and polysulfide, with linked sulfur atoms, have been found in vitro and in vivo.11) H2S is known to modify the enzyme proteins through the formation of a polysulfide and persulfide bond, and regulation of such activity by sulfhydration of enzyme.12,13)
In the present study, we examined the differences of vulnerability for oxidative stress in astrocytes of SHR and SHRSP brains. We also investigated the differences in the production of anti-oxidative substances including glutathione (GSH), H2S produced by CBS, and sulfane sulfur in astrocytes of SHR and SHRSP.
Acutase, Dulbecco’s modified Eagle’s medium (DMEM), penicillin-streptomycin mixed solution, 0.25% Trypsin solution and Alamar Blue (TM) were obtained from Nacalai Tesque (Kyoto, Japan). Fetal bovine serum (FBS) was bought from Corning (NY, U.S.A.). NucleoSpin RNA II was purchased from TaKaRa-Bio (Shiga, Japan). High-Capacity cDNA Reverse Transcription kits were purchased from Applied Biosystems Japan (Tokyo, Japan). LightCycler TaqMan Master was purchased from Roche (Tokyo, Japan). The antibody for CBS was purchased from Cell Signaling Technology Japan (Tokyo, Japan). Aminooxyacetic acid (AOAA) was bought from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). GSSG/GSH Quantification Kit and GYY4137 were purchased from Dojindo (Kumamoto, Japan). Glucose oxidase (GOD) was bought from Oriental Yeast Co., Ltd. (Tokyo, Japan). ThiolTracker Violet was purchased from Invitrogen (MT, U.S.A.). SulfoBiotics Sulfane Sulfur Probe 4 (SSP4) was kindly provided by Dojindo. Sulfidefluor-7 acetoxymethyl ester (SF7-AM) was kind gift from Dr. Alexander R. Lippert (Southern Methodist University, TX, U.S.A.).
SHRSP and SHR were supplied by the Disease Model Cooperative Study Association (Kyoto, Japan). Astrocyte preparation was carried out as described by McCarthy and de Vellis and Yamagata et al.14,15) Astrocytes from the cerebrum in 1- or 2-d-old SHR and SHRSP rats were cultured in DMEM supplemented with 10% FBS, 100 unit/mL penicillin and 100 µg/mL streptomycin, at 37°C in a CO2 incubator (95% air and 5% CO2). Cultures with a homogeneous cell population (consisting of >95% astrocytes as determined by glial fibrillary acidic protein staining) were used for the experiments.
Measurement of Cell Viability by GOD Using Alamar Blue (TM)Astrocytes were plated on a 96-well plate and incubated at 37°C until sub-confluency. The culture medium was changed to high glucose DMEM with 1% FBS with or without GOD, a reagent that sustains release of H2O2 from glucose followed by incubation at 37°C for 3 h. After 3 h, the cell culture medium was changed to phenol red free DMEM with 1% FBS and 10% Alamar Blue (TM) and incubated at 37°C for 3 h. Finally, the fluorescence intensity was measured (λex=560 nm, λem=595 nm) using the fluorescence plate reader and the viability was calculated.
Measurement of Total Thiols with ThiolTracker VioletA 5 mM stock solution of ThiolTracker violet (fluorescent probes for the thiol group including GSH and thiol group of protein) was prepared with dimethyl sulfoxide (DMSO). Cells were seeded on a 6-well plate and cultured at 37°C for 24 h. They incubated with 5 µM ThiolTracker violet at 37°C for 30 min and washed with phosphate-buffered saline without Ca2+ and Mg2+ (PBS(−)). Fluorescence was observed using a fluorescence microscope (Keyence, Osaka, Japan) (λex=404 nm, λem=526 nm) with randomly taken photographs. The fluorescence intensity was quantified using BZ-X Analyzer (Keyence) for 885–1712 cells per view.
Quantification of GSHCells were seeded on 6-well plates and cultured at 37°C for 24 h and washed with PBS(−), collected using 0.25% trypsin, centrifuged and collected again. To destroy the cell membrane, 1 mM HCl was added and freeze-thawing was repeated twice. For deproteination, 5% sulfosalicylic acid was added to the cell lysate, followed by centrifugation. The sulfosalicylic acid concentration was diluted to 0.5% with MilliQ water. Total GSH, reduced form of GSH and glutathione disulfide (GSSG) were measured according to the protocol of GSSG/GSH Quantification Kit by Dojindo. The values were corrected by the protein concentration of the cell lysates. In this study, protein quantification was done using Bio-Rad Protein Assay, based on the method of Bradford.
Quantification of H2S in AstrocytesSulfidefluor-7 acetoxymethyl ester (SF7-AM) is a dye that enters cells and fluoresces when it combines with hydrogen sulfide.16) A 5 mM stock solution of SF7-AM was prepared with DMSO. Astrocytes were seeded on a 96-well plate and cultured until subconfluent. They were then incubated with 5 µM SF7-AM at 37°C for 30 min and washed with PBS(−) according to a previously described method.17) Fluorescence was measured using a fluorescence plate reader (λex=485 nm, λem=535 nm). The fluorescence was corrected with protein concentration of the cell lysates.
Total RNA Extraction and Reverse Transcription (RT)–Real Time PCRTotal RNA was isolated from SHR and SHRSP astrocytes. Cultured cells were washed with ice cold PBS(−) and total RNA was extracted using NucleoSpin RNA II. Next, 2 µg of total RNA was transcribed into cDNA using High-Capacity cDNA Reverse Transcription kits, according to the manufacturer’s protocols. Real-time PCR was performed using LightCycler TaqMan Master (Applied Biosystems Assay ID: ribosomal protein L13A (RPL13a); Rn00821946_g1, CBS; Rn00560948_m1). The amplification reaction was monitored using a LightCycler nano (Roche Diagnostics, Tokyo, Japan). Expression values were determined using the ΔΔCT equation and corrected with expression levels of Ribosomal Protein L13a as a housekeeping gene.
Western Blot Analysis for Quantification of CBS ProteinAstrocyte samples were prepared in RIPA buffer (50 mM Tris–HCl (pH 7.6), 150 mM NaCl, 1% Nonidet®P 40 and 0.5% Sodium Deoxy Cholate) with protease and phosphatase inhibitors (Nacalai Tesque, Kyoto, Japan). Protein samples (30 µg/lane) were separated in 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred to a polyvinylidene difluoride (PVDF) membrane (ATTO, Osaka, Japan). The membranes were blocked with EZ-block Chemi (ATTO) and washed with Tris-buffered saline with 1% Tween. After blockage and probing with primary antibodies against CBS at 1 : 1000, followed by secondary rabbit immunoglobulin G (IgG) antibodies (CST, Tokyo, Japan) the signals were developed with ECL reagent (Nacalai Tesque) and then recorded using CCD camera system; Ez-CaptureII (ATTO). The results were quantifiably assayed with a CS Analyzer 3.0 (ATTO). The expressions of CBS protein were corrected using the expression of β-actin.
CBS Enzyme ActivityCBS activity was measured as an in vitro enzymatic reaction using SF7-AM. The astrocytes were lysed with 50 mM phosphate buffer (pH 8.2). One mg of protein was put in a 96-well plate and the fluorescent probe (5 µM SF7-AM) was preloaded at 37°C for 20 min. After 20 min, the fluorescence was measured (λex=485 nm, λem=535 nm) using a fluorescence plate reader and used as a blank. Next, 1 mM L-cysteine, 1 mM DL-homocysteine and 0.2 mM pyridoxal-5′-phosphate were added to the cells to start the enzyme reaction, followed by treatment with or without 1 mM AOAA as a CBS inhibitor. The plate was incubated at 37°C for the enzyme reaction. The fluorescence was measured every 30 min and the CBS activity was calculated. The details of calculation are listed in Results.
Effect of GYY4137 on Cell Death by H2O2 Oxidative StressGYY4137, a reagent that sustainably releases H2S,18) was developed by Moore and colleagues GYY4137 (Dojindo) when added at 100 µM, led to the release of 20 µM H2S. Astrocytes were plated on a 96-well plate and cultured until subconfluent. Cells were incubated with 0.1 unit/mL GOD with or without 20, 50 and 100 nM GYY4137 in DMEM with 1% FBS at 37°C for 3 h. After 3 h, the medium was changed to 1% FBS in colorless DMEM supplemented with Alamar Blue (TM) and incubated at 37°C for 3 h. Finally, the fluorescence was measured (λex=560 nm, λem=595 nm) using a fluorescence plate reader.
Quantification of Sulfane Sulfur in Astrocytes Using SSP4SSP4 is a dye that enters cells and fluoresces when it combines with sulfane sulfur. Cells were seeded on a 96-well plate and cultured until subconfluent. They were incubated with 10 µM SSP4 and 0.5 µM hexadecyltrimethylammonium bromide into DMEM without FBS at 37°C for 15 min according to a protocol by Dojindo. After 15 min, cells were washed with PBS(−) and fluorescence were measured using fluorescence plate reader (λex=485 nm, λem=535 nm). To investigate the participation of CBS, the cells were treated with 1 and 2 mM AOAA as a CBS inhibitor and pre-incubated at 37°C for 1 h. The fluorescence was corrected by the protein concentration of the cell lysates.
StatisticsData were analyzed by one-way ANOVA or t-test. In all cases, p<0.05 or p<0.01 was taken to indicate a significant difference.
Alamar Blue (TM) is a reagent for evaluating cells viability. Astrocytes in SHRSP were more vulnerable than SHR. Cell death of astrocytes was observed more at 0.02 unit/mL of GOD for SHRSP, but SHR astrocytes did not die at 0.1 unit/mL (Figs. 1a, b). The astrocytes from SHRSP are more vulnerable to H2O2 oxidative stress, possibly due to the impaired of scavenging ROS.

Values are indicated as the mean±S.E. (n=4–5). The cell viability is shown as a percentage of 0 unit/mL GOD group of SHR (a) and SHRSP (b). * p<0.05 and compared with the 0 unit/mL GOD group using William’s test.
Intracellular thiols play an important role in maintaining reduced conditions in the cells.
ThiolTracker violet, a fluorescent probe for intracellular thiols, reacts actively with reduced thiols including GSH in live cells. The total thiol levels in the SHRSP astrocytes were significantly lower than those of the SHR (Fig. 2). We therefore measured GSH. The total amount of GSH and the reduced form of GSH in SHRSP were not significantly different. However, the oxidized form of GSSG in SHRSP was significantly higher than in SHR. Therefore, GSH/GSSG in SHRSP was significantly lower than in SHR (Fig. 3). These results show that the cause of the decreased total thiols in SHRSP was not due to a decrease in GSH.
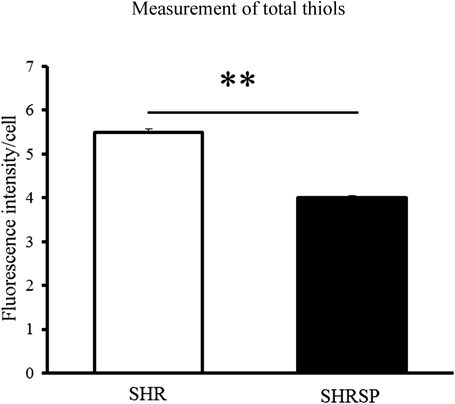
The fluorescence intensity for thiol levels per cell is indicated as the mean±S.E. (n=885–1712). ** p<0.01 compared with SHR using t-test. White column is SHR, black column is SHRSP.

White column is SHR, black column is SHRSP. The amount of total GSH is shown in (a), the reduced form of GSH (nmol/mg protein) in (b), the oxidized form of GSH in (c) and the ratio of GSH and GSSG in (d). Values for levels of GSH in cells are indicated as the mean±S.E. (n=3). * Significant at p<0.05 versus SHR using t-test.
We measured H2S, known to have a reducing effect in cells, in astrocytes from SHR and SHRSP. SF7-AM is a fluorogenic reagent designed and synthesized to detect H2S.16) It has been engineered specifically for use in live cell imaging of H2S. In quantifying H2S using SF7-AM, the value per protein was found to be significantly decreased in SHRSP astrocytes compared with those of SHR (Fig. 4). As H2S is produced by CBS in astrocytes, we next investigated the reduction of H2S in SHRSP caused by the expression of CBS or reduction of CBS enzyme activity.

White column is SHR, black Column is SHRSP. Fluorescence levels were corrected with protein concentration. Values are indicated as the mean±S.E. (n=4). ** Significant at p<0.01 versus SHR using t-test.
We investigated the expression of CBS known to be specifically expressed in astrocytes of the brain cortex and to synthesize the H2S. CBS mRNA expression levels in SHRSP astrocytes were significantly lower than those from SHR using real time PCR (Fig. 5a). Expressions of CBS protein in astrocytes were also significantly reduced in SHRSP compared with SHR (Fig. 5b).

White column is SHR, black column is SHRSP. mRNA expressions of CBS in (a) were corrected by the amount of RPL13a mRNA for ribosomal protein. CBS protein expression levels in (b) were corrected by expression of β-actin. Values are indicated as the mean±S.E. (n=3). ** Significant at p<0.01 and * significant at p<0.05 versus SHR using t-test.
The intensity of the fluorescence of SF7-AM bound with H2S increased linearly up to the measurement period of 1 h. Thus, CBS activity was calculated using the values at 1 h. CBS enzyme activity was calculated by subtracting the portion due to the AOAA CBS inhibitor. The result was that the CBS activity in SHRSP was significantly lower than in SHR (Fig. 6).

The values of CBS activity are shown as the fluorescence intensity without AOAA group−with AOAA group. White column is SHR, black column is SHRSP. The CBS activity in cells are indicated as the mean±S.E. (n=5). * Significant at p<0.05 versus SHR using t-test.
SHRSP astrocytes were vulnerable to H2O2 oxidative stress. Treatment with 0.1 unit/mL GOD injured the astrocytes, but 20 nM GYY4137 led to rescue from cell death at a significant level. High concentration groups of GYY4137 did not result in rescue from cell death (Fig. 7).

The graph shows the effect of GYY4137 for astrocyte cell death by GOD. Values are indicated as the mean±S.E. (n=4–5). White column is non-treatment group, black column is treatment of GOD 0.1 unit/mL group. The gray columns are GOD 0.1 unit/mL+ GYY4137 each. ** Significant at p<0.01 versus GOD 0.1 unit/mL group using Tukey test.
SSP4 is a fluorescent dye which is bound with sulfane sulfur without H2S. The amount of sulfane sulfur was significantly lower in SHRSP than SHR (Fig. 8a). Treatment with AOAA dose-dependently and significantly decreased the fluorescence intensity in SHR and SHRSP astrocytes (Figs. 8b, c).

Values of fluorescence intensity/protein shows the mean±S.E. (n=5). * Significant at p<0.05 versus SHR using t-test. The white column is SHR, black column is SHRSP. The amount of sulfane sulfur in astrocytes in (a) and with AOAA CBS inhibitor for 1h in (b–c). Values for levels of fluorescence intensity of SSP4 bound sulfane sulfur in cells are indicated the mean±S.E. (n=5). ** Significant at p<0.01 versus control using Dunnet test.
We showed the vulnerability of astrocytes in SHRSP to H2O2 oxidative stress. Astrocytes were exposed to the continuously released H2O2 from GOD with glucose in the culture medium. GOD catalyzes the oxidation of glucose to produce H2O2. SHRSP astrocytes died at lower concentrations of GOD than SHR.
Cellular redox balance is maintained by cellular thiols mainly GSH. The amount of total thiols in SHRSP was significantly lower than SHR. Total and reduced GSH showed lower tendency in SHRSP astrocytes, but not significant. However GSH/GSSG significantly decreased in SHRSP astrocytes compared with those from SHR. The decreased tendency of total GSH in SHRSP astrocytes may be related to CBS expression, because CBS is partly related with GSH synthesis.19) The ratio of GSH to GSSG is critical to cellular redox balance,17,20) the significant decreased ratio of GSH to GSSG cannot rule out of vulnerability in SHRSP astrocytes for oxidative stress.
Recent studies suggest that H2S has many physiological roles in processes, such as cytoprotection through an anti-oxidant effect or anti-inflammation and vasodilatation.5–8) In SHRSP astrocytes, production of H2S was significantly decreased compared with that in SHR.
The reduction in SHRSP astrocytes was caused by decreased expression of mRNA, protein and enzyme activity of CBS. CBS has been found in regions of the brain in particular localized in astrocytes. In the brain, CBS contributes mainly to the production of H2S using cysteine and homocysteine as a substrate.9,21) It is important for the metabolism of homocysteine. Lack of CBS and gene abnormality in humans causes hyperhomocysteinemia with a tendency to develop thrombosis, cardiac infarction and cerebral infarction.22) In this study, expressions of CBS and enzyme activity were both decreased in SHRSP. CBS in Dahl salt sensitive rats is regulated by hypoxia induced factor-1 (HIF-1).23) There was no difference in HIF-1 expression between SHR and SHRSP astrocytes (data not shown). Further, CBS gene sequences were observed no difference between the two strains (data not shown). We could not explain the cause of CBS gene suppression in SHRSP at present.
SHRSP rats are extensively used as a model of human stroke, but there have been few studies using SHRSP brain tissue. SHR also develops hypertension but without the onset of stroke. Yamori et al. showed that cerebral blood flow in SHRSP decreases following by elevated blood pressure.1–3) Reduction of blood flow causes hypoxia, subnutrition and induction of oxidative stress.
H2S also regulates the cerebral microvascular response to hypoxia. Some reports showed that under normal O2 condition, CO binds to the heme in CBS and inhibits the activity of producing H2S.24) Hypoxia induces production of H2S through decline of the generation of CO and vasodilation.4) As explained above, the cerebral blood flow reduces in SHRSP when blood pressure elevates. Ischemia induces hypoxia subsequently hypoxia causes production of ROS.25) In condition of hypoxia, CBS is activated and enhanced production of H2S, and it induces vasodilatation.26) The reduction of H2S in SHRSP may cause not enough vasodilation in condition of hypoxia. This decreased CBS expression may contribute to stroke-proneness in SHRSP.
H2S has been reported to modify specific cysteine residues in proteins through the formation of a polysulfide and persulfide bond and to be involved in the regulation of such activity by sulfhydration of the enzyme.10–13,27) The amount of sulfane sulfur was significantly lower in SHRSP astrocytes than in those of SHR. Sulfane sulfur was further reduced by the AOAA CBS inhibitor. AOAA is not a CBS-specific inhibitor, also inhibits cystathionine γ-lyase (CSE). CBS was thought to be the major H2S-producing enzyme in the brain. The expression of CBS was significantly decreased in SHRSP compared to SHR. Recent study reported that biologically active sulfur species are synthesized by CBS.28) We speculate that decreased CBS expression in SHRSP is related to production of sulfane sulfur.
We would like to thank Dojindo for the gift of SulfoBiotics SSP4. We are grateful to Dr. Alexander R. Lippert (Southern Methodist University, TX, U.S.A.) for generously providing the SF7-AM.
The authors declare no conflict of interest.