2016 Volume 39 Issue 12 Pages 1948-1954
2016 Volume 39 Issue 12 Pages 1948-1954
The effect of fungichromin (FC) on the formation of Candida albicans biofilm was assessed using 2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction method, scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM). Results showed that FC revealed an inhibitory effect on the formation of C. albicans biofilm in a dose-dependent manner with a minimum inhibitory concentration (MIC) of 10 µg/mL. Over 80% of biofilm formation was inhibited by FC at the concentration of 40 µg/mL when compared with the control. Similarly, real-time PCR showed that the expression of the genes such as ALS1, ALS3, HWP1, EFG1, HYR1, CPH1 and BCR1 appeared to be remarkably affected by FC at the concentration of 20 µg/mL during the biofilm formation. In addition, FC could also induce the apoptosis of C. albicans cells in a dose-dependent manner. Therefore, FC displayed potent activity against the formation of C. albicans biofilm in vitro and played an important role in reducing the incidence of device-associated infections.
Candida albicans is a dimorphic yeast that can be either a commensal or an opportunistic pathogen with the capability to cause a variety of infections, ranging from superficial mucous membrane infection to life-threatening systemic diseases.1,2) Invasive candidiasis carries unacceptably high mortality, which is reported to be as high as 40–60%.3) One of the major reasons for this phenomenon is that candidiasis can adapt to a variety of environmental conditions through attaching to the surface and growing in microbial community in a mode of biofilm.4) Biofilm can be formed not only on host tissues, but also on prosthesis and indwelling medical devices, such as urinary and vascular catheters, (dental) implants, prostheses, and heart valves.5,6) C. albicans biofilm can lead to a significantly increased resistance to conventional antifungal drugs.7) Another important reason for the low success rate in treating candidiasis is due to the limited number of antifungal agents. Also, the treatment efficacy of antifungal agents is limited because of toxicity and resistance.8,9)
Fungichromin (FC), as one of polyene antibiotics, has been reported for its in vitro antifungal properties including C. albicans10–12); however, until now, the activity of FC against sessile cells in C. albicans biofilm has never been studied. The aim of the present study is to explore the inhibitory effect of FC on the formation of C. albicans biofilm so as to evaluate the potential therapeutic implication for biofilm-associated candidal infections.
C. albicans was prepared using broth dilution technique. FC with the gradient concentration of 0.625–80 µg/mL was prepared by two-fold serial dilution technique through RPMI 1640 medium buffered to pH 7.0 with MOPS buffer, and then inoculated with 100 µL of C. albicans suspension. Fluconazole (Pfizer Inc., U.S.A.) and Amphotericin B (AmB) (Sigma Chemical Company, U.S.A.) were used as the controls, and fungichromin were prepared from the flesh mycelia of Streptomyces sp. WP. After 48 h incubation at 35°C, MICs were evaluated. The MIC of fluconazole was observed as the concentration that resulted in an 80% reduction of fungal growth when compared with the control. The MICs of FC and AmB were noted as the concentrations that resulted in complete visual inhibition of growth. MFC was determined by sub-culturing a 10 µL of the medium taken from the tubes which were showing no macroscopic growth after 48 h of Sabouraud Dextrose Agar Medium (1% Peptone, 4% Glucose, 1.5% Agar, pH 5.6±0.2) plates. The plates were incubated and scored for the growth of yeast colonies. MFC was the lowest concentration of the anti-fungal agent from which negative growth or fewer than 3 colonies were recorded.13)
Biofilm Formation and 2,3-Bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide (XTT) Reduction AssayC. albicans (ATC C 90028) was inoculated into YPD (1% yeast extract, 2% Bacto-Peptone, and 2% glucose) and grown overnight at 30°C with constant shaking (200 rpm). Cells were harvested and washed twice in sterile phosphate-buffered saline (PBS), and then added to RPMI 1640 to a concentration of 106 cells/mL. Biofilm experiments were performed in untreated 96-well cell culture plates. Totally 100 µL of cell suspension (106 cells/mL) in RPMI 1640 were introduced into each well for 1 h adhesion at 37°C. The wells were washed with PBS twice to remove non-adherent cells. Fresh RPMI 1640 containing drugs with various concentrations was added, followed by an additional incubation period at 37°C for 48 h. The quantity of biofilm in each well was assessed using a XTT reduction assay (Sigma-Aldrich, U.S.A.).14–16) Briefly, a saturated solution of XTT (0.5 mg/mL) was prepared, aliquoted, and stored at −70°C. Before XTT was used, menadione (10 mM in acetone) was added to a final concentration of 1 µM. Then, 100 µL of XTT-menadione solution was added to each well. The plates were incubated in dark at 37°C for 2 h and the colorimetric change at 490 nm was measured with a microtiter plate reader.
Confocal Laser Scanning Microscopy (CLSM)For CLSM,17) the cells were plated in the cell culture dish with a glass bottom in the presence or absence of samples after 1 h adhesion and 48 h incubation. Then, the cells were washed twice with PBS and stained with propidium iodide (PI, 15 µM) for 5 min at room temperature. After washing with PBS, the cells were incubated with fluorescein isothiocyanate (FITC)-conjugated Concanavaline A (ConA, 25 µg/mL) for 5 min at r.t. The cells were then washed in PBS and demineralized water, and analyzed with an Olympus fluorescence microscope.
Scanning Electron Microscopy (SEM)For SEM,18) the cells were cultured for another 48 h in 24-well cell culture plates with catheters in the presence or absence of samples after 1 h adhesion. Catheters were then harvested and placed in fixative (4% formaldehyde and 1% glutaraldehyde in PBS) overnight. Catheters were then rinsed with PBS and placed in 1% osmium tetroxide for 30 min. Catheters were dehydrated with a series of ethanol (30, 50, 70, 95, 100%), and final desiccation was completed by critical-point drying. Catheters were mounted, coated with gold and viewed under a scanning electron microscope (JEOL JSM-6100, Japan).
Relative Quantification by Real-Time Reverse Transcriptase (RT)-PCRThe expression of the genes associated with biofilm formation was evaluated using real-time PCR19) after treatment with FC, FLC and AmB. The cells were incubated with the samples at various concentrations for 48 h after 1 h adhesion. Total RNA was isolated with RNAPure (Peqlab Biotechnologie, Erlangen, Germany) according to the manufacturer’s instructions and converted to cDNA using RT through ReverTraAce (Toyobo Co., Osaka, Japan). For PCR detection of transcripts, a modified protocol was used to optimize the amplification of different primer sets. Primers used in this assay were shown in Table 1. The cDNA samples were subjected to preliminary denaturation at 95°C for 3 min, followed by 40 amplification cycles with the denaturation at 95°C for 7 s, annealing at 57°C for 10 s, and extension at 72°C for 15 s. The 5.8 s RNA was served as the internal control and the transcript level of tested genes was calculated using the formula 2−(ΔΔCt).
| Samples | Activity of FC, AmB and fluconazole against C. albicans | |
|---|---|---|
| MIC (µg/mL) | MFC (µg/mL) | |
| FC | 10 | 20 |
| AmB | 1 | 4 |
| Fluconazole | 0.3 | — |
—: No determination.
Apoptosis was evaluated using Annexin V-FITC/PI Apoptosis Detection Kit (BD Biosciences, San Diego, CA, U.S.A.).20) Then, the cells were incubated with the samples at various concentrations after 1 h adhesion and 48 h incubation. The adherent and floating cells were harvested and washed with PBS and re-suspended in 1× binding buffer at a concentration of 1×10 cells/mL. The cells were treated with Annexin V-FITC in dark for 30 min, and then incubated with propidium iodide for 2 min and immediately analyzed by flow cytometry using a FACSCalibur flow cytometer (Becton-Dickinson, Fullerton, CA, U.S.A.).
The inhibitory activity of FC against C. albicans has been reported.10) In this test, MICs and MFCs of FC, AmB and fluconazole for C. albicans were determined. FC exhibited an obvious inhibition on the growth of C. albicans at a dose-dependent manner. FC at 10 µg/mL, AmB at 1 µg/mL and fluconazole at 0.3 µg/mL could inhibit the growth of C. albicans, while FC at 20 µg/mL and AmB at 4 µg/mL could kill C. albicans (Table 1). Compared with fluconazole and AmB, FC also exhibited good inhibitory effect on the growth of C. albicans.
Inhibition of C. albicans Biofilm Formation with FCThe formation of C. albicans biofilm has been accepted as a key mechanism for the growth and survival of C. albicans in the host.21) Therefore, we initially detected serially double-diluted concentrations of FC against the formation of C. albicans biofilms by XTT reduction assay. Here, we used two strains (ATC C 90028 and CMCC (F) 98001), ATC C 90028 was usually used for biofilm investigation in many researches and CMCC (F) 98001 was also usually used for testing the activity of samples.22–25) As shown in Fig. 1(A), FC both had inhibitory effect on the two biofilm formations and the effects revealed a dose-dependent manner, respectively. We found that over 80% of biofilm formation was inhibited by FC at the concentration range of 40–80 µg/mL, while a lower concentration (20 µg/mL) could produce approximately 55% inhibitory effect on the formation of C. albicans biofilm; while AmB could produce approximately 60% inhibitory effect on the formation of C. albicans biofilm at the concentration of 20 µg/mL in ATC C 90028 biofilm formation. We could also find that the similar effect of FC on CMCC(F) 98001 biofilm formation showed in Fig. 1(B).

Cells were treated with FC (2.5–80 µg/mL) after 1 h adhesion. Crystal violet assessment of C. albicans biofilm formation after 1 h growth in RPMI 1640 treated with FC at various concentrations (2.5–80 µg/mL) was also conducted. (A) C. albicans biofilm formation: ATCC 90028; (B) C. albicans biofilm formation: CMCC(F) 98001. All data are expressed as the mean±standard error (S.E.) from three independent experiments.
To further confirm the efficacy of FC on inhibition of the biofilm formation, the samples were double-stained with red fluorescent PI (binding to DNA) and green fluorescent ConA-FITC.26) CLSM showed that normal C. albicans biofilm exhibited a typical three-dimensional structure. The positive control cells treated with 150 ng/mL fluconazole showed an obvious inhibition on biofilm development. When the cells were treated with 20 µg/mL FC, biofilm development was obviously inhibited and revealed poor architecture. Many C. albicans was even dead at 30 µg/mL FC. CLSM showed the decreased thickness of the biofilm as the increase of drug concentration (Fig. 2).

Concanavalin A (green) and PI (red) staining were used to generate the images. (A) Normal biofilm showed a three-dimensional structure. (B) When the cells were treated with 150 ng/mL fluconazole after 1 h incubation, the biofilm development was inhibited. (C, D, E) When the cells were treated with 10, 20 and 30 µg/mL FC at 1 h incubation, the biofilm development was inhibited at different degrees.
C. albicans biofilm formation on catheters was monitored by SEM. The pre-treatment of C. albicans with different samples showed that FC and fluconazole had high potential to inhibit hyphal growth of C. albicans. Wherever, the hyphae were intact in the negative control. Partial cell wall treated with FC at a concentration of 20 µg/mL was damaged (Fig. 3).

(A) Control: biofilm-associated adherent cells without drugs. (B) Fluconazole: biofilm-associated adherent cells with fluconazole at the concentration of 150 ng/mL. (C) FC: biofilm-associated adherent cells with FC at the concentration of 20 µg/mL.
To explore the mechanism of FC for inhibiting biofilm formation, the selected genes such as ALS1, ALS3, HWP1, EFG1, HYR1, CPH1 and BCR127–32) (Table S1) of biofilm-associated C. albicans cells and planktonic cells were measured by real-time PCR. Biofilm-associated adherent cells were cultured with drugs (fluconazole at the concentration of 150 ng/mL, AmB at the concentration of 10 µg/mL, and FC at the concentrations of 20, 10 µg/mL) or without drugs. Planktonic cells were cultured in YPD without drugs. The genes including ALS1, ALS3, HWP1, EFG1, HYR1, CPH1 and BCR1 were overexpressed from C. albicans associated with biofilm formation without drugs when compared with planktonic cells (p<0.01). Fluconazole and AmB as the positive control could remarkably affect the expression of genes (ALS1, ALS3, HWP1, EFG1, HYR1, CPH1 and BCR1) in biofilm (p<0.05 or p<0.01). Similarly, the expression of genes (ALS1, ALS3, HWP1, EFG1, HYR1, CPH1 and BCR1) appeared to be remarkably affected by FC at the concentration of 20 µg/mL in biofilm (p<0.05 or p<0.01) (Fig. 4). When the cells were treated with 10 µg/mL FC in biofilm, the expression of genes (HWP1, EFG1, HYR1, CPH1 and BCR1) was also increased (p<0.05); in contrast, the genes (ALS1 and ALS3) did not reveal difference when compared with nature biofilm.

Control: biofilm-associated adherent cells without drugs. Fluconazole: biofilm-associated adherent cells with fluconazole at the concentration of 150 ng/mL; AmB: biofilm-associated adherent cells with AmB at the concentration of 10 µg/mL; FC (20 µg/mL) and FC (10 µg/mL): biofilm-associated adherent cells with FC at the concentration of 20 and 10 µg/mL, respectively; Planktonic: planktonic cells cultured in YPD without drugs. Results are the mean±standard error (S.E.) from three independent experiments. *** p<0.01, * p<0.05, compared with the control cells.
FITC-Annexin V staining, which binds to phosphatidylserine with high affinity in the presence of Ca2+, could examine the early stage of apoptosis. Propidium iodide (PI) is the membrane-impermeable dye for the staining of nuclei.20) In order to determine the type of FC-induced cell death, the FITC-Annexin V and PI double-staining methods were used. The cells with FC at the concentrations of 10, 20, and 30 µg/mL showed a significant increase in the population of apoptotic cells including Annexin V+/PI− (early stage of apoptotic cells) and Annexin V+/PI+ (late stage of apoptotic cells), suggesting that FC treatment could induce the early apoptosis. As shown in Fig. 5, compared with the positive control AmB, FC treatment could induce the similar apoptosis rate. The apoptosis in FC-treated biofilm-associated C. albicans also showed a dose-dependent manner.
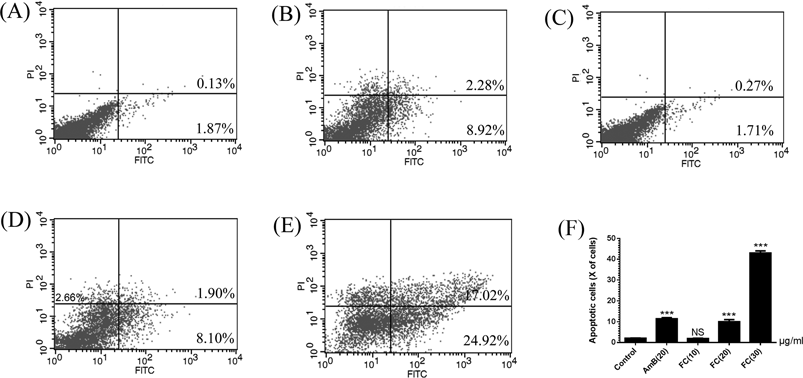
(A) Control: biofilm-associated adherent cells without drugs; (B–E) FC: biofilm-associated adherent cells with AmB at the concentrations of 20 µg/mL or FC at the concentrations of 10, 20 and 30 µg/mL, respectively. Apoptotic cells were those stained with Annexin V-FITC+/PI− (early apoptotic cells) and Annexin V-FITC+/PI+ (late apoptotic cells). (F) The percentages of apoptotic cells were presented in bar charts. *** p<0.01, compared with the control cells.
Biofilm is a protective environment for C. albicans, serving as a protective barrier against the host immune system and antifungal drugs. C. albicans can attach to the surface of devices and proliferate as a biofilm including massive amounts of extracellular matrix.33) Biofilm shows a reduced sensitivity to drugs including fluconazole, itraconazole, nystatin and chlorhexidine when compared with planktonic organisms.19) As the rapid progression of drug resistance, novel and effective products should be developed for the control of candidiasis. FC, as a macrolide compound, has been reported to have a wide range of antifungal activities.10–12) Here, its impact on C. albicans biofilm formation is first investigated.
In the present study, FC has confirmed to be an active drug against planktonic C. albicans. The 48-h MIC and MFC of FC for planktonic C. albicans ATC C 90028 cells were 10 and 20 µg/mL, respectively. During exploring the effect of FC on C. albicans biofilm formation by XTT reduction method, CLSM and SEM,19,21,28) FC reveals a well inhibition on biofilm formation in a dose-dependent manner.
The formation of C. albicans biofilm includes a series of sequential steps: adhesion, initiation, maturation, and dispersal.33) ALS gene family, the member encoding cell-wall glycoproteins, is an important factor for the adhesion of C. albicans.34,35) It has been reported that ALS1 gene is expressed in the earlier stage of biofilm formation,36) and ALS3 gene is mainly up-regulated in the mature biofilm.37) HWP1 gene could also regulate the adhesion of C. albicans to epithelial cells by Hwp1 protein.38) BCR1, as a positive regulator of adherence, was required for the expression of many cell surface protein genes such as ALS1, ALS3, and HWP1.39,40) The transformation of hyphae morphology plays an important role in biofilm formation and pathogenicity. HYR1 gene provides an important handle on morphogenetic regulation in C. albicans.41) EFG1 and CPH1 genes, as transcriptional regulators, have been identified as having key roles in controlling the growth of hyphae.42,43) The positive correlation between biofilm formation capacity and genes (ALS1, ALS3, HWP1, HYR1, EFG1, CPH1 and BCR1) has been found. The expression level of the genes (ALS1, ALS3, HWP1, HYR1, EFG1, CPH1 and BCR1) from biofilm is significantly higher than that from planktonic cells. The positive control AmB and fluconazole both showed the effect on expression of the genes tested. Compared with the positive control, FC also showed the effect on the expression of the genes (HWP1, HYR1, EFG1, CPH1 and BCR1) at the lower concentration and FC could show much better effect on the expression of the genes (ALS1, ALS3, HWP1, HYR1, EFG1, CPH1 and BCR1), genes (ALS1, HWP1, HYR1, EFG1 and CPH1) were even suppressed to less than 30% at the higher concentration. When compared with the positive control AmB, FC showed the similar effect on the expression of the genes (HWP1, HYR1, EFG1, CPH1 and BCR1) at the same concentration. Therefore, the genes (ALS1, ALS3, HWP1, HYR1, EFG1, CPH1 and BCR1) in biofilm cultivated with FC at various concentrations is inhibited as the increase of FC concentration, and the expression of the genes (ALS1, ALS3, HWP1, HYR1, EFG1, CPH1 and BCR1) reveals a decreasing trend or even a return to the normal level, suggesting that FC may down-regulate the expression of the genes (ALS1, ALS3, HWP1, HYR1, EFG1, CPH1 and BCR1) in biofilm. The change of mycelium-associated proteins can lead to the inhibition of C. albicans biofilm formation.
Apoptosis is a highly regulated cellular suicide program crucial for the development and homeostasis of metazoan organisms, even eukaryotes.33) C. albicans exposed to FC reveals the staining of Annexin V+/PI− and Annexin V+/PI+, suggesting the apoptosis in biofilm-associated cells.
In conclusion, compared with the classic polyene anti-fungal drug-AmB, FC could not only show the similar effect on the biofilm formation, but also had the similar effect on the expression of the genes (HWP1, HYR1, EFG1, CPH1 and BCR1). FC may down-regulate the expression of the genes (ALS1, ALS3, HWP1, HYR1, EFG1, CPH1 and BCR1) to inhibit the formation of C. albicans biofilm. FC can also promote the apoptosis in biofilm-associated C. albicans. Therefore, our results indicated that FC might be a new choice for the treatment of biofilm-associated candidal infections.
The present study was supported by National Science and Technology Major Project 2009ZX09301-011, Medical Guiding Project, Science and Technology Commission of Shanghai Municipality, China (Grant No. 134119a8700).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.