2016 Volume 39 Issue 2 Pages 289-294
2016 Volume 39 Issue 2 Pages 289-294
Sickness behavior is a series of behavioral and psychological changes that develop in those stricken with cancers and inflammatory diseases. The etiological mechanism of sickness behavior is not known in detail, and consequently there are no established standard therapies. Kamikihito (KKT), a Kampo (traditional Japanese herbal) medicine composed of 14 herbs, has been used clinically to treat psychiatric dysfunction. Previously, we found that KKT ameliorated sickness behavior in mice inoculated with murine colon 26 adenocarcinoma cells. In this study, we examined the effects of KKT on bacterial endotoxin lipopolysaccharide (LPS)-induced sickness behavior in mice. The administration of LPS caused the emotional aspects of sickness behavior, such as loss of object exploration, social interaction deficit, and depressive-like behavior. LPS also induced mRNA expression for cyclooxygenase (COX)-2, interleukin (IL)-1β and IL-6, and increased the number of c-Fos immunopositive cells in the hypothalamus and amygdala. KKT ameliorated the behavioral changes and reversed the increases in c-Fos immunopositive cells in the two brain regions, but did not influence the mRNA expression. These results suggest that KKT ameliorates sickness behavior via the suppression of neural activation without anti-inflammatory effects, and that KKT has the potential to treat sickness behavior.
Sickness behavior refers to a set of adaptive behavioral and psychological changes, such as fatigue, anhedonia, depression, anxiety, apathy and loss of appetite, that occur in patients with cancer or infections.1–3) Several studies have shown that activation of the immune system based on increases in release of inflammatory cytokines is associated with sickness behavior,2,4–13) but the etiological mechanism is not known in detail. Thus, there are no standard therapies for sickness behavior.
The mechanism of induction of sickness behavior by immune system activation has been examined in bacterial endotoxin lipopolysaccharide (LPS)-treated animal models. Administration of LPS induces neuroinflammation and neural activation in rodent brain, and causes sickness behavior.11,14) Neural activation in the paraventricular nucleus (PVN) of the hypothalamus and the central nucleus of the amygdala (CeA) and emotional aspects of behavioral changes such as depressive-like behavior, social behavior deficits and loss of interest continue for more than 24 h after LPS administration.14,15) These studies suggest that neural activation of the PVN and CeA is involved in LPS-induced sickness behavior.
Kamikihito (KKT) is a Kampo (Japanese herbal) medicine is used clinically to treat psychiatric dysfunctions such as anxiety, insomnia, amnesia and depression. Pharmacological studies have shown that KKT is effective for behavioral abnormalities and cognitive dysfunction induced by beta-amyloid, methyl-beta-carboline-3-carboxylate, scopolamine, delta-9-tetrahydrocannabinol, ovariectomy, and senescence,16–20) which suggests that KKT has an effect on the central nervous system (CNS). Recently, we found that KKT ameliorates emotional aspects of sickness behavior in murine colon 26 adenocarcinoma cells-inoculated mice.21)
In this study, we examined the effects of KKT on LPS-induced sickness behavior in mice, with the aim of evaluating the possible therapeutic utility of KKT in sickness behavior. To clarify the mechanism underlying the ameliorative effect of KKT on sickness behavior, we examined the effect of KKT on LPS-induced inflammation and neural activation in the hypothalamus and amygdala.
Experimental procedures concerning the use of animals were conducted according to the Guiding Principles for the Care and Use of Laboratory Animals approved by the Japanese Pharmacological Society and the committee for Ethical Use of Experimental Animals at Setsunan University. Every effort was made to minimize animal suffering and to reduce the number of animals used. Seven-week-old male ddY mice were obtained from Shimizu Laboratory Supplies Co., Ltd. (Kyoto, Japan) and housed in cages (24×17×12 cm) in groups of 5 animals under controlled environmental conditions (23±1°C; 12 : 12-h light–dark cycle, humidity of 55%, food and water ad libitum) for 1 week before use in experiments. We used different mice in each experiment.
Drug TreatmentsLPS (from Escherichia coli O127:B8; Sigma, St. Louis, MO, U.S.A.) was dissolved in saline (0.9% (w/v) solution of NaCl) and injected intraperitoneally (i.p.) 2 h before decapitation for mRNA measurement or 24 h before behavioral tests or decapitation for immunohistochemistry. The component herbs of KKT were indicated in Table 1. The Kampo formula was decocted with 600 mL of distilled water until the volume was reduced to half. The extract was immediately filtrated through filter paper in vacuo. The filtrate was lyophilized and the yield of KKT extract was approximately 27% of the herbal mixture, based on its dry weight. KKT was dissolved in distilled water and injected orally 1 h before LPS administration. All drugs were injected at a fixed volume of 10 mL/kg body weight. To identify the chemical constituents of KKT extract, liquid column chromatography-mass spectrometry analyses were performed as described in supplementary materials.
| The component herbs | The manufacturer | Lot No. | Amount |
|---|---|---|---|
| Ginseng Radix | Uchida Wakan-yaku Co., Ltd., Tokyo, Japan | 00F1204 | 3.0 g |
| Atractylodis Rhizoma | Tochimoto Tenkaido Co., Ltd., Osaka, Japan | 009313005 | 3.0 g |
| Poria | Tochimoto Tenkaido Co., Ltd., Osaka, Japan | 00951510016 | 3.0 g |
| Zizyphi Semen | Tochimoto Tenkaido Co., Ltd., Osaka, Japan | 021110007 | 3.0 g |
| Longan Arillus | Tochimoto Tenkaido Co., Ltd., Osaka, Japan | 022813003 | 3.0 g |
| Bupleuri Radix | Tochimoto Tenkaido Co., Ltd., Osaka, Japan | P011304241 | 3.0 g |
| Astragali Radix | Tochimoto Tenkaido Co., Ltd., Osaka, Japan | 001013005 | 3.0 g |
| Angelicae Radix | Tochimoto Tenkaido Co., Ltd., Osaka, Japan | P011212271 | 2.0 g |
| Gardeniae Fructus | Tochimoto Tenkaido Co., Ltd., Osaka, Japan | 004613004 | 2.0 g |
| Polygalae Radix | Tochimoto Tenkaido Co., Ltd., Osaka, Japan | 001410002 | 2.0 g |
| Zizyphi Fructus | Uchida Wakan-yaku Co., Ltd., Tokyo, Japan | 0301163 | 2.0 g |
| Glycyrrhizae Radix | Uchida Wakan-yaku Co., Ltd., Tokyo, Japan | 00F3255 | 1.0 g |
| Saussureae Radix | Tochimoto Tenkaido Co., Ltd., Osaka, Japan | 010513001 | 1.0 g |
| Zingiberis Rhizoma | Tsumura & Co., Tokyo, Japan | D41821 | 0.5 g |
| Total 31.5 g |
Each mouse was habituated in an observation cage (24×17×12 cm) for 15 min. After the habituation period, a novel object (a wooden ball of diameter 5 cm) was placed in the center of the cage and behaviors were videotaped for 5 min. The duration of object exploratory behavior (sniffing or licking the wooden ball) was measured.
Social Interaction TestA ddY mouse pretreated with drugs and an unfamiliar 8-week-old male ddY mouse were placed in a neutral cage (24×17×12 cm) and behaviors were videotaped for 20 min. The total duration of social behaviors (sniffing, licking and following the unfamiliar mouse) of the pretreated mouse was measured.
Forced Swim TestMice were individually placed in a polymethylpentene beaker (height 27 cm, diameter 18 cm) containing 25±1°C water of depth 13 cm. The performance of the mice for 6 min in the swimming session was videotaped. The total duration of immobility was measured in the final 4 min of the 6-min test session.
Locomotor ActivityLocomotor activity was measured using ANY-maze video tracking software (Stoelting Co., Wood Dale, IL, U.S.A.). Each mouse was placed in a novel opaque polyvinyl chloride cage (40×25×30 cm3). The behaviors of mice were videotaped for 30 min and the total distance traveled was analyzed.
Quantitative Real-Time Polymerase Chain Reaction (PCR)Total RNA was isolated from the hypothalamus and amygdala with TRIzol reagent (Invitrogen, Carlsbad, CA, U.S.A.). Total RNA (1 µg) was used to perform reverse transcription with ReverTra Ace (Toyobo, Osaka, Japan). Quantitative real-time PCR was performed using a Thermal Cycler Dice Real Time System Single (TaKaRa Bio Inc., Shiga, Japan) and the primers indicated in Table 2. Changes in gene expression were calculated relative to the endogenous β-actin standard.
| mRNA | Forward primer sequence (5′ to 3′) | Reverse primer sequence (5′ to 3′) |
|---|---|---|
| COX-2 | GGCCATGGAGTGGACTTAAA | GGGATACACCTCTCCACCAA |
| IL-1β | TGTGAAATGCCACCTTTTGA | CAGGTCAAAGGTTTGGAAGC |
| IL-6 | GTTCTCTGGGAAATCGTGGA | TTCTGCAAGTGCATCATCGT |
| β-Actin | ACCCACACTGTGCCCATCTA | GCCACAGGATTCCATACCCA |
The c-Fos immunohistochemistry was performed as previously described.15) Briefly, mice were anesthetized and perfused transcardially with saline, followed by a solution of 4% paraformaldehyde. The brain was fixed with 4% paraformaldehyde over 2 d. Serial 50-µm thick coronal sections containing the PVN (−0.8 to −1.0 mm with respect to the bregma) and CeA (−1.0 to −1.2 mm with respect to the bregma) were cut using a microslicer (DTK-1000, Dosaka EM Co., Ltd., Kyoto, Japan). The free-floating sections were subjected to immunostaining analysis using an anti-c-Fos rabbit polyclonal primary antibody (1 : 20000 dilution; Calbiochem, San Diego, CA, U.S.A.), a biotinylated anti-rabbit immunoglobulin G (IgG) secondary antibody (1 : 200 dilution; Vector Laboratories, Burlingame, CA, U.S.A.) and avidin-biotin-horseradish peroxidase complex (Vectastain ABC kit; Vector Laboratories). Brown cytosolic products were obtained by reaction with 3,3′-diaminobenzidine (Sigma, St. Louis, MO, U.S.A.). The number of c-Fos-positive nuclei were counted manually in three independent sections using a microscope (IX71, Olympus, Tokyo, Japan) with a CCD camera (VB-7010, Keyence, Osaka, Japan), and determined in a 500×500 µm2 area. The mean of this average across three sections was calculated for each mouse.
Statistical AnalysisAll data are expressed as the mean±standard error of the mean (S.E.M.). Data for behavioral tests and immunohistochemistry were analyzed using one-way ANOVA followed by a Tukey–Kramer post-hoc test. Data for mRNA expression were analyzed using two-way ANOVA followed by a Tukey–Kramer post-hoc test. Statistical analyses were performed using the software package, Statview 5.0 J for Apple Macintosh (SAS Institute Inc., Cary, NC, U.S.A.). A value of p<0.05 was considered to be significant.
LPS (500 µg/kg) decreased the novel object exploration and social interaction, and increased the immobility time in the forced swim test. These LPS-induced behavioral changes were present at 24 h after LPS administration. KKT (300 mg/kg) significantly reversed the decrease in novel object exploration and social interaction, and the increase in immobility time in the forced swim test (F3, 28=6.535, p=0.0017 for the object exploration test; F3, 33=4.614, p<0.0084 for social interaction test; F3, 31=7.615, p=0.0006 for the forced swim test). KKT (1000 mg/kg) also significantly reversed the decrease in novel object exploration and the increase in immobility time in the forced swim test, but did not influence the decrease in social interaction (Figs. 1A–C). Locomotor activity was not significantly changed at 24 h after LPS administration with or without treatment with KKT (F3, 24=0.770, p=0.5222) (Fig. 1D).
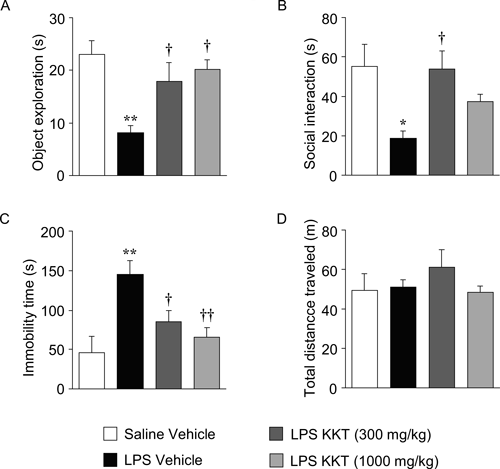
Mice were treated with LPS (500 µg/kg) 24 h before the behavioral tests. (A) Effect of KKT on LPS-induced exploratory behavior deficits. Each mouse was placed in an observation cage (24×17×12 cm). After 15 min, a novel object (a wooden ball of diameter 5 cm) was placed in the center of the cage and the duration of object exploratory behavior (sniffing or licking the wooden ball) was measured. Values are expressed as the mean±S.E.M. of 8 mice. (B) Effect of KKT on LPS-induced social interaction deficits. A mouse pretreated with drugs and an unfamiliar mouse were placed in a neutral cage (24×17×12 cm) and videotaped for 20 min. The total duration of social interaction behavior of the pretreated mouse was analyzed. Values are expressed as the mean±S.E.M. of 9–10 mice. (C) Effect of KKT on LPS-induced depressive-like behavior in the forced swim test. The total duration of immobility was measured in the final 4 min of a 6-min test session. Values are expressed as the mean±S.E.M. of 8-10 mice. (D) Effects of LPS and KKT on spontaneous locomotor activity. Each mouse was placed individually in a novel cage (40×25×30 cm) and the distance traveled was analyzed for 60 min. Values are expressed as the mean±S.E.M. of 7 mice. * p<0.05, ** p<0.01 vs. saline/vehicle-treated mice. † p<0.05, †† p<0.01 vs. LPS/vehicle-treated mice.
To investigate the mechanism underlying the influence of KKT on LPS-induced sickness behavior, we examined the effect of KKT on LPS-induced inflammation in the hypothalamus and amygdala. LPS (500 µg/kg) increased cyclooxygenase (COX)-2, interleukin (IL)-1β and IL-6 mRNA levels in the hypothalamus and amygdala at 2 h after LPS injection. In control mice, KKT had no effect or slightly decreased mRNA expression for inflammation-related genes in the hypothalamus and amygdala (Figs. 2A, B). Similarly, KKT did not affect the LPS-induced mRNA expression for inflammation-related genes in the hypothalamus or amygdala of sickness behavior model mice (Figs. 2A, B). Two-way ANOVA revealed main significant effects of LPS administration (F(1, 23)=22.5, p<0.0001 for hypothalamic COX-2; F(1, 23)=13.2, p<0.01 for hypothalamic IL-1β; F(1, 23)=27.3, p<0.0001 for hypothalamic IL-6; F(1, 23)=22.6, p<0.001 for amygdaloid COX-2; F(1, 23)=19.3, p<0.01 for amygdaloid IL-1β; F(1, 23)=31.2, p<0.0001 for amygdaloid IL-6) but not KKT treatment (F(1, 23)=0.4, p>0.05 for hypothalamic COX-2; F(1, 23)=0.7, p>0.05 for hypothalamic IL-1β; F(1, 23)=0.1, p>0.05 for hypothalamic IL-6; F(1, 23)=0.6, p>0.05 for amygdaloid COX-2; F(1, 23)=0.1, p>0.05 for amygdaloid IL-1β; F(1, 23)=1.4, p>0.05 for amygdaloid IL-6), and there was no significant interaction between LPS administration and KKT treatment (F(1, 23)=0.1, p>0.05 for hypothalamic COX-2; F(1, 23)=0.6, p>0.05 for hypothalamic IL-1β; F(1, 23)=0.1, p>0.05 for hypothalamic IL-6; F(1, 23)=0.5, p>0.05 for amygdaloid COX-2; F(1, 23)=0.1, p>0.05 for amygdaloid IL-1β; F(1, 23)=1.4, p>0.05 for amygdaloid IL-6). The increases in mRNA levels for inflammation-related genes were not observed at 24 h after LPS injection (data not shown).

Expression levels of COX-2, IL-1β and IL-6 mRNA in the hypothalamus (A) and amygdala (B) are shown as fold changes relative to levels in saline/vehicle-treated mice. Values are expressed as the mean±S.E.M. of 6 mice. * p<0.05, ** p<0.01 vs. saline-treated mice. †p<0.05 vs. vehicle-treated mice.
We previously found that neural activation in the PVN of the hypothalamus and the CeA are likely to be related to LPS-induced sickness behavior.15) Therefore, we examined the effect of KKT on LPS-induced expression of c-Fos, an indirect marker of neural activity,21) in these nuclei at 24 h after LPS injection. LPS induced significant increases in the number of c-Fos immunopositive cells in the PVN and CeA, and KKT (300 mg/kg) attenuated these increases (F2, 15=14.650, p=0.0003 for the PVN; F2, 15=33.040, p<0.0001 for the CeA) (Fig. 3).
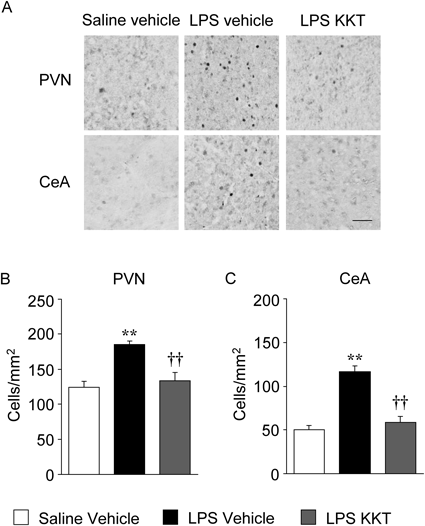
Immunohistochemical localization of the neural activity marker c-Fos was determined 24 h after LPS administration. (A) Representative photomicrographs showing c-Fos staining in the PVN (top panels) and CeA (bottom panels) in brain sections of mice treated with saline/vehicle (left panels), LPS/vehicle (center panels) and LPS/KKT (right panels). Scale bars, 100 µm. (B, C) Numbers of c-Fos immunopositive cells in the PVN (B) and CeA (C). Values are expressed as the mean±S.E.M. of 6 mice. ** p<0.01 vs. saline/vehicle-treated mice. †† p<0.01 vs. LPS/vehicle-treated mice.
Previous studies have reported that LPS-induced decreases in spontaneous locomotor activity and food intake are ameliorated within 10–16 h, while LPS-induced decreases in novel object exploration and social interaction and an increase in immobility time in the forced swim test remain after 24 h.14,22) Murine colon 26 adenocarcinoma cells-inoculated mice showed sickness behavior such as exploratory behavior deficit, social interaction deficits and depressive-like behavior, whereas locomotor activity and food intake were not affected.23) These findings suggest that decreases in novel object exploration and social interaction and an increase in immobility time in the forced swim test can be regarded as common symptoms between two murine experimental models of sickness behavior. In this study, KKT ameliorated the LPS-induced behavioral changes without affecting spontaneous locomotor activity (Fig. 1). We previously showed that KKT ameliorates emotional aspects of sickness behavior in tumor-inoculated mice without affecting spontaneous locomotor activity.21) These results suggest that KKT may have utility for treatment of core symptoms in sickness behavior.
Systemic administration of LPS triggers peripheral innate immune cells to secrete inflammatory cytokines such as IL-1β and IL-6.24) These peripheral inflammatory cytokines act on the brain and induce upregulation of inflammatory cytokine production in the CNS, which is associated with sickness behavior.25) In addition, an increase in prostaglandin synthesis through inflammation-induced COX-2 in the CNS is also implicated in sickness behavior.13,22,26,27) We previously reported that genipin, a main constituent of Gardeniae Fructus, is one of the component herbs of KKT, ameliorated LPS-induced sickness behavior and inflammation in the hypothalamus and amygdala 2 h after LPS administration.15) Therefore, it seemed likely that KKT would also attenuate LPS-induced inflammation in the hypothalamus and amygdala 2 h after LPS administration. Contrary to our expectation, KKT tended to decrease mRNA expression for inflammation-related genes in control mice, but did not reverse the LPS-induced increases in mRNA levels in either brain region (Fig. 2). These results suggest that the ameliorative effects of KKT on LPS-induced sickness behavior cannot be explained fully by anti-inflammatory effect.
In rodents, peripheral LPS injection activates several brain nuclei associated with sickness behavior, including the PVN and CeA, through the vagus nerve and the nucleus of the solitary tract.28,29) We have previously shown that LPS (500 µg/kg, i.p.) increased the number of c-Fos immunopositive cells in the PVN and CeA, and that these increases in c-Fos immunopositive cells were correlated with sickness behavior in ddY mice.15) In this study, we found that KKT reversed the increases in c-Fos immunopositive cells in both the PVN and CeA (Fig. 3), which implies that KKT ameliorates LPS-induced sickness behavior via suppression of immune response-induced neural activation. KKT may attenuate the neural activation by blocking the afferent vagus signals from peripheral sites of inflammation via the vagus nerve. Further studies are needed to clarify the mechanisms through which KKT prevents LPS-induced neural activation.
In conclusion, KKT ameliorated LPS-induced sickness behavior via suppression of neural activation in the PVN and CeA, but had little effect on LPS-induced inflammation. These results indicate that KKT has potential for treatment of sickness behavior. Our findings also suggest that suppression of neural activation in the PVN and CeA is a potential therapeutic approach for treatment of sickness behavior.
We wish to thank Mr. Ryosuke Yukioka, Ms. Kumi Okamoto and Ms. Yumi Kamikawatoko for their technical assistance.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.