Abstract
The therapeutic effects of anti-methicillin-resistant Staphylococcus aureus (MRSA) agents, vancomycin (VCM), teicoplanin (TEIC), and arbekacin (ABK), depend on their concentrations in blood. Therefore, therapeutic drug monitoring (TDM) is important when these antibiotics are used. In the hematological ward at Tokushima University Hospital, pharmacists have ordered the measurement of blood VCM, TEIC, and ABK concentrations to promote the use of TDM in accordance with an agreed protocol since 2013. Moreover, the infection control team includes several medical disciplines and has advised on the optimal treatment using VCM, TEIC, and ABK since 2013. This study aimed to investigate the clinical effectiveness of these pharmacist interventions. We retrospectively studied 145 cases in which patients were treated with VCM, TEIC, or ABK between January 2012 and December 2013 in the hematological ward at Tokushima University Hospital. The patients were divided into a control group (71 cases) and an intervention group (74 cases), and their clinical outcomes were compared. The rate of achievement of effective drug concentrations significantly increased in the intervention group (74%), compared to the rate in the control group (55%). Moreover, univariate and multivariate Cox proportional hazard regression revealed that pharmacist intervention and appropriate concentrations of anti-MRSA agents were independent factors associated with reduced hospitalization periods in patients with lymphoma. Our study revealed that proactive pharmacist intervention may improve the therapeutic effect of anti-MRSA agents in hematology ward patients.
Methicillin-resistant Staphylococcus aureus (MRSA) shows multi-drug resistance and is treated using vancomycin (VCM), teicoplanin (TEIC), arbekacin (ABK), linezolid (LZD), or daptomycin (DAP) in Japan. The therapeutic effects of VCM, TEIC, and ABK are dependent on their blood concentrations.1) Therapeutic drug monitoring (TDM) is an important consideration during their use, because low blood concentrations can result in treatment failure or the acquisition of drug resistance.
Pharmacists play an important role in TDM by advising physicians on the most effective treatment regimens. A previous study reported that pharmacist intervention using TDM in an intensive care unit optimized anti-MRSA therapy.2) This pharmacist intervention is also required on hematological wards, where multidrug chemotherapy is employed, particularly for leukemia and lymphoma treatments, and severe neutropenia and infection are often present.3) For this reason, anti-MRSA agents are used more frequently in these patients than in those with other diseases.4) However, there are few reports of the efficacy of pharmacist TDM interventions in this setting.
Protocol-based pharmacotherapy management (PBPM), where pharmacists manage pharmaceutical care in accordance with a general protocol agreed with the relevant physicians, was recently introduced in Japan.5) It is based on the collaborative drug therapy management employed in the United States, which has improved clinical outcomes.6) Moreover, the involvement of an inter-disciplinary medical support team can improve clinical efficacy by monitoring adverse drug reactions and recommending optimal treatment strategies.7) Hence, PBPM and the medical support team facilitate active pharmacist involvement in patient treatment.
Under PBPM, pharmacists in a hematological ward at the Tokushima University Hospital have been ordering blood VCM, TEIC, and ABK measurements since January 2013. At the same time, the infection control team (ICT) of ward pharmacists, physicians, nurses, and clinical technologists was formed, and it recommended the optimal treatments for patients receiving anti-MRSA agents. The present study investigated the clinical effects of pharmacist interventions through PBPM and ICT.
PATIENTS AND METHODS
PBPM in the Hematology WardWe created a drug therapy management protocol allowing pharmacists or physicians to order blood drug determinations. This protocol was mutually agreed by the physicians and pharmacists, and by the steering committee.
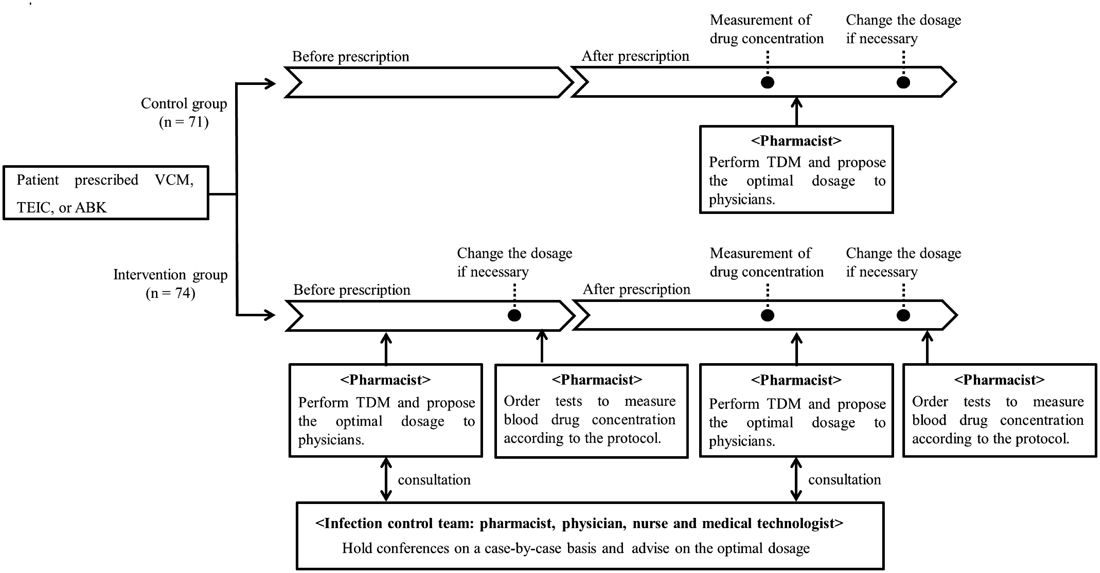
Pharmacist InterventionsIf administration of VCM, TEIC, or ABK was planned, pharmacists predicted blood drug concentrations using software (VCM: SHIONOGI-VCM-TDM S-edition ver. 2009, Shionogi Inc., Japan; TEIC: TEICTDM ver. 2.1, Sanofi Aventis Inc., Japan; ABK: ABK TDM analysis software ver. 2.0, Meiji Seika Pharma Inc., Japan) and proposed an optimal dosage to the physicians. If drug concentration control was predicted to be difficult, the pharmacists consulted the ICT, which advised on the optimal dosage. Blood drug concentration measurements were ordered by ward pharmacists or physicians. Pharmacists conducted TDM when they received the drug concentration results and proposed any necessary dose modifications. At this stage, the pharmacists could consult the ICT. If the dosage was changed, further blood drug determinations were ordered. This intervention scheme is shown in Fig. 1.
Patient CharacteristicsThis study was reviewed and approved by the Ethics Committee of Tokushima University Hospital. We analyzed the medical records of patients who received VCM, TEIC, or ABK between January 2012 and December 2013 in the Tokushima University Hospital hematology ward. Patients were excluded if antimicrobial administration was stopped for 3 d, if they were aged <20 years, or if creatinine clearance was <30 mL/min, which would probably affect drug elimination; 145 patients were enrolled. These were divided into the control group (no pharmacist involvement) and the intervention group (with pharmacist involvement). We collected retrospective clinical information from medical records. Creatinine clearance was calculated as previously described.8) Methicillin-resistant Staphylococcus species (MRS) carrier cases were defined as those where MRS had been detected before or during the administration of anti-MRSA agents. Febrile neutropenia (FN) was defined as an axillary temperature of ≥37.5°C at a neutrophil count of <500 cells/µL or <1000 cells/µL with a predicted decline to <500 cells/µL.9)
Effects of the Pharmacist Interventions on Anti-MRSA TreatmentWe calculated the antimicrobial use density (AUD) as previously described.10) No patients in this survey were diagnosed with severe infection and the optimal concentrations were defined as: VCM and TEIC, trough concentration of 10–20 µg/mL; ABK, trough concentration of <2.0 µg/mL and peak concentration of 9–20 µg/mL.11–13) Achievement was recorded if the optimal drug concentration was reached once during treatment. We checked the incidence of acute renal injury, defined as an increase in serum creatinine to ≥1.5 times baseline after drug administration.14) Defervescence was defined as an axillary temperature of <37.5°C during all days.
StatisticsThe clinical laboratory values were not normally distributed and the Mann–Whitney U-test was therefore used by to analyze the proportional scales. χ2 or Fisher’s exact test was used to analyze the nominal scales. The hospitalization period was compared by Kaplan–Meier plots. Univariate and multivariate Cox proportional hazard analyses were carried out to analyze the effect of pharmacist intervention on the hospitalization period. The factors identified as significantly different by univariate analysis were used in multivariate analysis. All analyses were conducted using Excel (Microsoft). All recorded p values were two-sided, and those <0.05 were considered statistically significant.
RESULTS
Patient CharacteristicsTable 1 shows the patient characteristics in the two groups. VCM, TEIC, and ABK were administered to 38, 32, and 30% of the control group and 47, 37, and 16% of the intervention group, respectively; there were no significant differences between these proportions in the two groups. The reasons for anti-MRSA agent administration, cancer type, treatment history, and clinical laboratory values did not differ significantly between the study groups.
Table 1. Patient Characteristics
| Control | Intervention | p Value |
|---|
| No. of cases | 71 | 74 | |
| Vancomycin (%) | 27 (38) | 35 (47) | 0.34a) |
| Teicoplanin (%) | 23 (32) | 27 (37) | 1.00a) |
| Arbekacin (%) | 21 (30) | 12 (16) | 0.09a) |
| Age, median (range), (years) | 53 (24–78) | 50 (25–80) | 0.08b) |
| Male: female | 47 : 24 | 43 : 31 | 0.41a) |
| Carrier of MRS (%) | 30 (42) | 25 (34) | 0.15a) |
| Reason for anti-MRSA agent administration |
| Febrile neutropenia (%) | 57 (80) | 60 (81) | 1.00a) |
| Treatment of MRS (%) | 8 (11) | 8 (11) | 1.00a) |
| Suspected MRS infection (%) | 6 (9) | 6 (8) | 1.00a) |
| Leukemia |
| Chemotherapy (%) | 33 (47) | 33 (45) | 0.95a) |
| Allogeneic stem cell transplantation (%) | 19 (27) | 15 (20) | 0.47a) |
| Lymphoma |
| Chemotherapy (%) | 11 (15) | 13 (18) | 0.91a) |
| Autologous peripheral blood stem cell transplantation (%) | 3 (4) | 6 (8) | 0.49c) |
| Other disease (%) | 5 (7) | 7 (9) | 1.00c) |
| White blood cell, median (range) (×103/µL) | 0.4 (0.1–8.5) | 0.4 (0.1–8.0) | 0.25b) |
| C-Reactive protein, median (range) (mg/dL) | 5.3 (0.08–22.5) | 5.7 (0.3–19.2) | 0.97b) |
| Albumin, median (range) (g/dL) | 2.8 (1.5–4.1) | 3.0 (1.3–4.2) | 0.14b) |
| Aspartate aminotransferase, median (range) (IU/L) | 17 (6–102) | 17 (5–81) | 0.72b) |
| Alanine aminotransferase, median (range) (IU/L) | 24 (2–86) | 16 (5–90) | 0.38b) |
| Creatinine clearance, median (range) (mL/min) | 93.2 (32.4–180.6) | 115.3 (30.5–199.8) | 0.16b) |
Data are expressed as the median (range) or number (%). a) χ2 test; b) Mann–Whitney U test; c) Fisher’s exact test. Abbreviations: MRS, methicillin-resistant Staphylococcus; MRSA, methicillin-resistant Staphylococcus aureus.
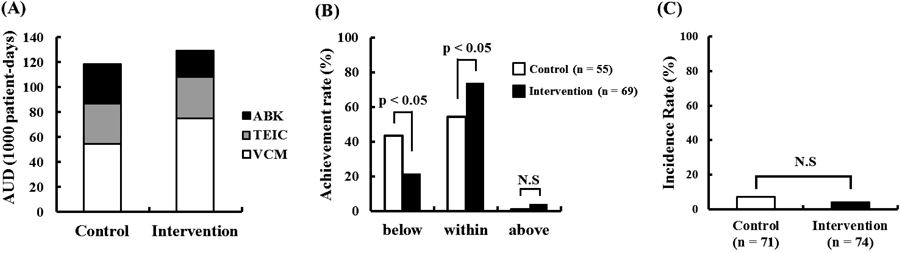
The AUD (1000-patient days) of each anti-MRSA agent used in the control and intervention groups, respectively, was as follows: VCM, 55 and 75; TEIC, 31 and 35; and ABK, 32 and 21 (Fig. 2A). The optimal drug concentration achievement rate was significantly higher in the intervention group (74%) than in the control group (55%) (Fig. 2B). No differences in the incidence of acute renal injury were observed between the control (7%) and intervention (4%) groups (Fig. 2C).
Table 2 shows the effect of the intervention on anti-MRSA agent usage. The initial dose and actual drug concentration of TEIC were significantly higher in intervention groups. Days until the blood concentration test and the duration of anti-MRSA agent administration did not differ significantly between these groups. The defervescence rate 3 d after anti-MRSA agent administration in lymphoma patients was significantly higher in the intervention group. The median hospitalization period of all patients was 70 d in the control group and 47.5 d in the intervention group (Fig. 3A). The corresponding periods for leukemia patients was 70 versus 135 d, and 61 versus 35 d for lymphoma patients; this difference was significant for the lymphoma patients (Figs. 3B, C). Univariate and multivariate Cox proportional hazard analyses revealed that appropriate concentrations of anti-MRSA agents (hazard ratio: 0.46, 95% confidence interval (CI): 0.18–0.89) and pharmacist intervention (hazard ratio: 0.43, 95% CI: 0.20–0.92) were independent factors associated with a reduced hospitalization period in lymphoma patients (Table 3).
Table 2. Effect of Pharmacist Intervention on Anti-MRSA Agent Usage
| Control | Intervention | p Value |
|---|
| Initial dose, median (range) |
| Vancomycin (mg) | 1000 (500–2000) | 1000 (500–2000) | 0.55a) |
| Teicoplanin (mg) | 400 (200–400) | 400 (200–800) | 0.018a) |
| Arbekacin (mg) | 200 | 200 | NA |
| Actual drug concentration, median (range) |
| Vancomycin (µg/mL) | 9.5 (5.2–22.1) | 11.7 (4.4–29.2) | 0.10a) |
| Teicoplanin (µg/mL) | 8.7 (4.3–17.7) | 11.5 (6.8–17.1) | 0.014a) |
| Arbekacin |
| Trough concentration (µg/mL) | <0.8 (<0.8–1.3) | <0.8 (<0.8–2.7) | 0.20a) |
| Peak concentration (µg/mL) | 10.7 (7.9–17.4) | 15.0 (8.6–18.7) | 0.12a) |
| Days until blood test, median (range) | 4 (3–16) | 4 (3–9) | 0.062a) |
| Duration of anti-MRSA agent administration, median (range) | 11 (4–18) | 10 (4–14) | 0.38a) |
| Defervescence rate 3 d after administration (%) | 20 (28) | 25 (34) | 0.58b) |
| Leukemia patients (%) | 18 (33) | 15 (31) | 0.88b) |
| Lymphoma patients (%) | 2 (14) | 10 (53) | 0.032c) |
a) Mann–Whitney U test; b) χ2 test; c) Fisher’s exact test. Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus, NA, not analyzed.
Table 3. Cox Proportional Hazard Regression Analysis for the Hospitalization Period in Lymphoma Patients
| Factors | Univariate analysis | Multivariate analysis |
|---|
| Hazard ratio | 95% CI | p Value | Hazard ratio | 95% CI | p Value |
|---|
| Use of VCM | 1.15 | 0.50–2.63 | 0.75 | — | — | — |
| Carrier of MRS | 1.39 | 0.69–2.88 | 0.36 | — | — | — |
| Febrile neutropenia | 0.99 | 0.41–2.38 | 0.96 | — | — | — |
| Age | 1.04 | 0.99–1.08 | 0.06 | — | — | — |
| Male | 1.12 | 0.56–2.27 | 0.74 | — | — | — |
| Autologous peripheral blood stem cell transplantation | 1.16 | 0.45–2.70 | 0.73 | — | — | — |
| White blood cell | 0.96 | 0.76–1.22 | 0.76 | — | — | — |
| C-Reactive protein | 1.03 | 0.95–1.11 | 0.46 | — | — | — |
| Albumin | 0.97 | 0.91–1.04 | 0.41 | — | — | — |
| Aspartate aminotransferase | 1.01 | 0.99–1.03 | 0.36 | — | — | — |
| Alanine aminotransferase | 1.01 | 0.99–1.03 | 0.44 | — | — | — |
| Creatinine clearance | 1.01 | 0.98–1.02 | 0.17 | — | — | — |
| Adequate concentration of anti-MRSA agents | 0.43 | 0.20–0.93 | 0.032 | 0.46 | 0.18–0.89 | 0.026 |
| Pharmacist intervention | 0.46 | 0.23–0.94 | 0.035 | 0.43 | 0.20–0.92 | 0.029 |
Abbreviations: CI, confidence interval; VCM, vancomycin; MRS, methicillin-resistant Staphylococcus; MRSA, methicillin-resistant Staphylococcus aureus.
DISCUSSION
The present study revealed that pharmacist intervention through PBPM and ICT collaboration was associated with the facilitation of adequate anti-MRSA agent TDM and a reduced hospitalization period in lymphoma patients. Previous studies have demonstrated the effectiveness of pharmacist intervention in patients receiving anti-MRSA agents.3,15) However, most reported interventions were initiated after the prescription of anti-MRSA agents. The present intervention was initiated prior to prescription using PBPM. Our findings indicated that appropriate pharmacist intervention in the ICT before the initial prescription reduced the hospitalization period in lymphoma patients. There are no previous reports showing the clinical effectiveness of this approach and we propose that these interventions provide a new strategy for pharmacist intervention in hematological disease.
Although this intervention reduced the hospitalization period for lymphoma patients, this was not observed in the leukemia patients, where a chronic and severe neutropenia is induced by chemotherapy and the primary disease. Chemotherapy-induced neutropenia is less severe during lymphoma treatment. Moreover, 53% of the lymphoma patients in the intervention group received TEIC. Blood TEIC concentration was significantly higher in intervention group in our study. These differences in neutropenia and drug concentration may have contributed to the specific reduction in the hospitalization period for lymphoma patients.
In our study, all patients received broad-spectrum antimicrobial and antifungal agents. Therefore, antimicrobials other than anti-MRSA agents probably did not affect our results. The ABK AUD was lower in the intervention group. This may be due to the lower number of patients prescribed ABK in this group. In contrast, the AUDs of VCM and TEIC were higher in the intervention group. This increase reflected pharmacist-initiated increases in VCM and TEIC dosages to increase the drug concentration, based on TDM. However, we did not identify any effect of the intervention on the duration of anti-MRSA agent prescription. This may be because the majority of patients who were administered anti-MRSA agents were diagnosed with FN and the administration was prolonged until the recovery of neutropenia, even if defervescence was observed.
The anti-MRSA agents, LZD and DAP, are also used for FN treatment.16) None of the patients included in the present study were administered DAP. LZD was administered to 27 patients in the control group and 7 patients in the intervention group (p<0.01, χ2 test). The therapeutic effect of the pharmacist intervention on the hospitalization period may be associated with this decreased LZD usage.
There were two aspects to the intervention applied in the present study. The first was the introduction of PBPM. In the control group, there were 16 cases (23%) where no measurements of drug concentration were conducted. However, this was significantly lower in the intervention group (5 cases, 7%) since there were 16 cases (23%) in which pharmacists (instead of physicians) ordered blood concentration determinations. These results showed that pharmacist-driven PBPM facilitated the proper TDM of anti-MRSA agents. The second aspect of the intervention was the pharmacist-ICT collaboration. Before this study was conducted, a report system was introduced in our hospital in order to limit the inappropriate use of anti-MRSA agents. However, the pharmacist or ICT were not involved in the treatment until after the initial drug prescription. In this study, the hematological ward pharmacist participated in the ICT. The links between the ward pharmacist and the ICT facilitated sharing of patient information and intervention prior to the initial prescription. This proactive collaboration between the pharmacists and the ICT may have contributed to the observed improvements. However, we could not assess the individual contributions of PBPM or the ICT collaboration to our results. We speculate that both approaches were necessary to achieve the present results.
There were several limitations to this retrospective study. First, the study was restricted to only one ward, limiting the patient population to the hematological field. Second, the minimum inhibitory concentration for MRS was not measured and we could not assess the drug sensitivity of the MRS present in this population. Third, the optimal drug concentration was not achieved in 15 intervention group patients (22%). Moreover, TDM was not conducted in 5 intervention group cases because either the ward pharmacist was absent or the patient was not identified as receiving anti-MRSA agents. These results indicated that our intervention was insufficient and stricter pharmacist intervention might be required to produce more effective outcomes.
CONCLUSION
In conclusion, the present study showed that pharmacist interventions through PBPM and ICT facilitated the application of TDM for anti-MRSA agents. Moreover, proactive pharmacist interventions and the achievement of the appropriate drug concentrations were associated with shorter hospitalization periods in lymphoma patients. Although further prospective analyses with more patients are needed to confirm these findings, the present study provided useful information supporting the value of pharmacist interventions.
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- 1) Begg EJ, Atkinson HC, Jeffery GM, Taylor NW. Individualised aminoglycoside dosage based on pharmacokinetic analysis is superior to dosage based on physician intuition at achieving target plasma drug concentrations. Br. J. Clin. Pharmacol., 28, 137–141 (1989).
- 2) Imaura M, Kohata Y, Kobayashi K, Takahashi H, Yokoyama H, Akase T, Yamada Y. Effect of pharmacists’ intervention on the antibiotic therapy for the methicillin-resistant Staphylococcus aureus (MRSA) infectious diseases in the intensive care unit. Yakugaku Zasshi, 131, 563–570 (2011).
- 3) Arizumi H, Maeda T, Nakashima H, Hattori N, Yanagisawa K, Saito B, Harada H, Nakamaki T, Mori H, Tomoyasu S. A descriptive epidemiological study of hematological disorders in Showa University Hospital. J. Showa Univ. Society, 73, 224–231 (2013).
- 4) Terui Y. Strategies of treatment for febrile neutropenia. Gan To Kagaku Ryoho, 40, 688–692 (2013).
- 5) Sugimoto M, Yonezawa A, Tadehara M, Morita Y, Yoshida Y, Onoue M, Omura T, Kayano Y, Fukatsu S, Yano I, Matsubara K. Protocol-based pharmacotherapy management (PBPM) involving ward-pharmacists as a member of medical team on outpatient prescriptions brought to the hospital. Jpn. J. Pharm. Health Care Sci., 40, 297–303 (2014).
- 6) Isetts BJ, Schondelmeyer SW, Heaton AH, Wadd WB, Hardie NA, Artz MB. Effects of collaborative drug therapy management on patients’ perceptions of care and health-related quality of life. Res. Social Adm. Pharm., 2, 129–142 (2006).
- 7) Kajizono M, Aoyagi M, Kitmura Y, Sendo T. Effectiveness of medical supportive team for outpatients treated with sorafenib: a retrospective study. J. Pharm. Health Care Sci., 1, 39–44 (2015).
- 8) Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron, 16, 31–41 (1976).
- 9) Masaoka T. Evidence-based recommendations for antimicrobial use in febrile neutropenia in Japan: Executive summary. Clin. Infect. Dis., 39 (Suppl. 1), S49–S52 (2004).
- 10) Matsumoto K, Shigemi A, Yaji K, Shimodozono Y, Takeda Y, Ikawa K, Morikawa N, Miyanohara H, Orita M, Tokuda K, Nishi J, Yamada K. Reduction in the incidence of MRSA with use of alcohol-based hand rub solutions and gloves. J. Infect. Chemother., 18, 269–271 (2012).
- 11) Matsumoto K, Takesue Y, Ohmagari N, Mochizuki T, Mikamo H, Seki M, Takakura S, Tokimatsu I, Takahashi Y, Kasahara K, Okada K, Igarashi M, Kobayashi M, Hamada Y, Kimura M, Nishi Y, Tanigawara Y, Kimura T. Practice guidelines for therapeutic drug monitoring of vancomycin: A consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J. Infect. Chemother., 19, 365–380 (2013).
- 12) Ueda T, Takesue Y, Nakajima K, Ichki K, Wada Y, Tsuchida T, Takahashi Y, Ishihara M, Tatsumi S, Kimura T, Ikeuchi H, Uchino M. Evaluation of teicoplanin dosing designs to achieve a new target trough concentration. J. Infect. Chemother., 18, 296–302 (2012).
- 13) Okada K, Kimura T, Mikamo H, Kasahara K, Seki M, Takakura S, Tokimatsu I, Ohmagari N, Takahashi Y, Matsumoto K, Igarashi M, Kobayashi M, Hamada Y, Mochizuki T, Kimura M, Nishi Y, Tanigawara Y, Takesue Y, Japanese Society of Chemotherapy, Japanese Society of Therapeutic Drug Monitoring. Clinical practice guidelines for therapeutic drug monitoring of arbekacin: A consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug monitoring. J. Infect. Chemother., 20, 1–5 (2014).
- 14) Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, Kellum JA. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit. Care, 10, R73 (2006).
- 15) Welty TE, Copa AK. Impact of vancomycin therapeutic drug monitoring on patient care. Ann. Pharmacother., 28, 1335–1339 (1994).
- 16) Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR, Infectious Diseases Society of America. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis., 52, 427–431 (2011).