2016 Volume 39 Issue 3 Pages 308-312
2016 Volume 39 Issue 3 Pages 308-312
Pulmonary carcinoma is a major cause of cancer-related death worldwide. Because the prognosis remains poor, the development of novel therapeutic approaches is highly desirable. In this study, we investigated the effect of Tamibarotene (Am80), a retinoic acid derivative, on the growth of human lung adenocarcinoma cell line A549. Our ultimate goal in this study is to provide pulmonary carcinoma therapy with a new approach. First, we treated A549 cells with Am80 to clarify the effect of cell-growth inhibition. Am80 significantly reduced the viability of A549 cells in a dose- and time-dependent manner. The IC50 value, which was determined using CellTiter-Glo Luminescent Cell Viability assay, of Am80 and all-trans retinoic acid (ATRA) against A549 cells at 6 d was 49.1±8.1 µM and 92.3±8.0 µM, respectively. Furthermore, Am80 reduced the anchorage-independent cell-growth ability of A549 cells. However, it was not an apoptosis-mediated mechanism. These results suggest that Am80 can be used as an effective, novel cell-growth inhibitor in lung adenocarcinoma.
Pulmonary carcinoma is a major cause of cancer-related death worldwide.1) Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all pulmonary carcinoma cases. This cancer is categorized into histological subtypes of adenocarcinoma, squamous cell carcinoma, and large cell carcinoma.2) Because the prognosis of NSCLC remains poor, the development of novel therapeutic approaches is highly desirable.3) Lung adenocarcinoma, accounting for approximately 40% of all pulmonary carcinomas, is currently one of the most common histological types and its incidence has gradually increased in recent years in many countries.4)
Conventional chemotherapeutic regimens against most solid tumors have reached an efficacy plateau and display significant toxicity. In the last decade, the targeted inhibition of oncogenic driver mutations with molecular therapies, of which the epidermal growth factor receptor (EGFR) and the anaplastic lymphoma kinase (ALK) are the most studied targets, has brought dramatic improvements in the overall survival in defined subsets of patients.5) Unfortunately, in many cases, patients develop resistance to these agents via secondary mutations and alternative mechanisms.6)
In this study, we focused on a retinoic acid derivative as a cell-growth inhibitor in lung adenocarcinoma. Retinoids can inhibit the growth and modulate the differentiation of a variety of tumor cell types in vitro and in vivo.7) A novel synthetic retinoid, Tamibarotene (Am80), has been developed and applied as acute promyelocytic leukemia treatment. Am80 offers higher differentiation-inducing property and is approximately ten times more potent than all-trans retinoic acid (ATRA) as an in vitro inducer of differentiation in NB-4 and HL-60 leukemia cells.8,9) In addition, Am80 is chemically more resistant to light, heat, and oxidation and has higher receptor selectivity compared with ATRA. Am80 is a synthetic selective agonist of nuclear retinoic acid receptor (RAR)-α and RAR-β and does not bind to RAR-γ, which is the major retinoic acid receptor in the dermal epithelium.10) In terms of metabolic properties, Am80 is resistant to the retinoic acid-metabolizing enzyme CYP26A1 and weakly binds to cellular retinoic acid-binding protein (CRABP), which might lead to ATRA resistance.11–13) The affinity of Am80 for CRABP is only about one-twentieth of ATRA affinity. Although Am80 has clear advantages over ATRA, its antiproliferative effect on pulmonary carcinoma has not been studied.
To evaluate the applicability of Am80 as a cell-growth inhibitor in pulmonary carcinoma, with the ultimate goal to provide pulmonary carcinoma therapy with a new approach of differentiation-inducing therapy, we examined its effect on the viability of A549 human pulmonary carcinoma cells.
Am80 was kindly provided by Itsuu Laboratory (Tokyo, Japan). Am80 was dissolved in ethanol and stored at −30°C until use. ATRA (Wako Pure Chemical Industries, Ltd., Osaka, Japan) was also dissolved in ethanol and stored at −30°C until use. Antibodies against CRABP-I (cat. sc-10061), CRABP-II (cat. sc-10065), actin (cat. sc-1616) and donkey anti-goat immunoglobulin G-horseradish peroxidase (IgG-HRP) (cat. sc-2020) were obtained from Santa Cruz Biotechnology Inc. (U.S.A.). Nonfat dry milk was obtained from Cell Signaling Technology, Inc. (U.S.A.). All polymerase chain reaction (PCR) primers were purchased from Eurofins MWG Operon Inc. (Germany).
Cell Lines and Cell CultureThe established human pulmonary carcinoma cell line A549 was provided by Terada laboratory in Tokyo University of Science. A549 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Wako Pure Chemical Industries, Ltd.) supplemented with 10% fetal bovine serum (FBS), at 37°C in a humidified 5% CO2 atmosphere. The established human lung fibroblast cell line MRC-5 was provided by Nishioka Laboratory in Tokushima University Graduate School. The MRC-5 cell line was derived from normal lung tissue of a 14 weeks old male fetus in 1966 by J.P. Jacobs. MRC-5 cells cultured in Eagle’s Minimal Essential Medium (EMEM; Wako Pure Chemical Industries, Ltd.) supplemented with 10% FBS, non-essential amino acids, 1 mM sodium pyruvate, and 1500 mg/L sodium bicarbonate at 37°C in a humidified 5% CO2 atmosphere.
Cell Viability AssayThe cell viability of A549 or MRC-5 cell lines treated with Am80 or ATRA was assessed using the CellTiter-Glo Luminescent Cell Viability Assay (Promega KK, Tokyo, Japan), according to the manufacturer’s protocol. Cells were seeded at 625 per cm2 in 100 µL of medium in 96-welled flat-bottom plates and grown overnight at 37°C in an incubator. After exposure to Am80 for 6 d, the plates were assayed using EnVision Plate Reader (PerkinElmer, Inc. Japan Co., Ltd., Kanagawa, Japan).
Western Blot AnalysisCells were cultured in 90 mm plates to confluency, washed with phosphate buffered saline (PBS), and lysed in 2× sample buffer [0.5 M Tris(hydroxymethyl)aminomethane hydrochloride (Tris–HCl), pH 6.8, with 10% sodium dodecyl sulfate (SDS), 2-mercaptoethanol, glycerol and bromophenol blue (BPB)] and denatured at 95°C for 10 min. Following centrifugation, protein concentrations in cell lysates were determined by the Bradford assay (Bio-Rad). Equivalent total protein amounts were loaded in each lane of an SDS–18% polyacrylamide gel electrophoresis (PAGE) gel, followed by transfer to a polyvinylidene fluoride (PVDF) membrane and blocking in 5% nonfat dry milk. Blots were probed with appropriate primary antibodies, followed by incubation with horseradish peroxidase-conjugated secondary antibody (donkey anti-goat IgG-HRP) and visualized by ImageQuantLAS4000EPUVmini (GE Healthcare UK Ltd., U.K.). The antibodies were diluted with Canget signal immunostain (Toyobo, Japan).
Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR)The RT-PCR method described previously was adopted with some modifications. Total RNA was extracted using RNAiso reagent (TaKaRa, Japan), and 0.05-µg aliquots of RNA were reverse-transcribed (RT) using a ReverTra Ace quantitative polymerase chain reaction (qPCR) RT kit (Toyobo, Japan). The cDNA equivalent of 1 ng of input RNA was amplified by real-time PCR (Applied Biosystems; Life Technologies, Carlsbad, CA, U.S.A.). Sequences for PCR primers are listed in Table 1.
| Gene | Sequences | |
|---|---|---|
| CRABP-II | Forward | 5′-GCTTTCTTTGACCTCTTCTCTC-3′ |
| Reverse | 5′-GGCTAGGACTGCTGACTTG-3′ | |
| GAPDH | Forward | 5′-GCACCGTCAAGGCTGAGAAC-3′ |
| Reverse | 5′-TGGTGAAGACGCCAGTGGA-3′ | |
Cells were seeded at 3125 per cm2 and grown 48 h at 37°C in an incubator. After exposure to Am80 for 1 d, the plates were fixed with 4% paraformaldehyde in PBS. The cells were stained according to the manufacturer’s protocol of the kit (in situ Apoptosis Detection Kit, TaKaRa, Japan). The slides were rinsed in PBS three times and examined under a fluorescence microscope (Keyence, Japan). The apoptosis rate was calculated by number of TUNEL-positive cells/500 DAPI-positive cells.
Colony Formation AssayCells were plated at 625 per cm2 in DMEM containing 10% FBS. The following day, Am80 was added to the cells and the culture was incubated for 6 d. Subsequently, A549 cells were harvested, suspended in a medium containing 0.35% soft agar, and seeded upon the base layer at a density of 750 cells per well. Base layer was made by mixing 1% soft agar and the equivalent volume of 2× medium. After 2 weeks, the colonies were counted under a microscope. Cells were stained with crystal violet solution.
Statistical AnalysisNormally distributed data are presented as the mean±standard deviation (S.D.) or standard error (S.E.). Student’s t-test was used when only two groups were compared. p-Values <0.01 or <0.05 were considered statistically significant.
Am80 reduced the viability of A549 cells in a dose-dependent manner. The inhibition ratios of 50, 100, and 200 µM Am80 were approximately 50, 89, and 99.6%, respectively (Fig. 1). Cell-growth inhibition was observed in a time-dependent manner after a 6-d treatment with 50 µM Am80 and after a 2-d treatment with 100 and 200 µM Am80 (Fig. 1). Am80 also inhibits the cell growth in other cancer cell line, such as human breast cancer cells and the human glioblastoma cell line.14,15)

CellTiter-Glo assay in A549. A549 cell line (625 cells/cm2) was incubated with or without Am80 (50, 100, and 200 µM) for 2–6 d after 24-h pre-culture. The medium containing Am80 was exchanged every 48 h. Results represent the mean±S.D. of n=3 experiments. * p<0.05, ** p<0.01 vs. control treatment group for each time point. For plots of cell survival rate, the mean basal value (day 0) of each group was set at 100%.
ATRA also reduced the viability of A549 (Fig. 2), although Am80 has higher potential for antiproliferative effect comparison with ATRA.

A549 cell line (625 cells/cm2) was incubated with or without ATRA or Am80 (1, 10, 50, 100, and 200 µM) for 6 d after 24-h pre-culture. The medium containing drug was exchanged every 48 h. Results represent the mean±S.D. of n=3 experiments. ** p<0.01 ATRA vs. Am80 treated group for each dose. † <0.01, †† <0.01 vs. control treatment group for each dose: † is ATRA and †† is Am80. For plots of cell survival rate, the mean basal value (Control) of each cell line group was set at 100%.
Expression levels of CRABP-II protein were evaluated by Western blot analysis. Treatment with 10 µM Am80 for 60 min showed an increase in the expression of CRABP-II protein (Figs. 3A, B). In addition, expression levels of CRABP-II mRNA were evaluated by qRT-PCR. Treatment with 50 µM Am80 for 6 d showed an increase in the expression of CRABP-II mRNA (Fig. 3C).

(A, B) Influence of Am80 on expression of CRABP-II protein levels in A549 cells. The data were normalized using β-actin as a Control. (C) Influence of Am80 on expression of CRABP-II mRNA levels in A549 cells. The data were normalized using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a Control. Results represent the mean±S.D. of n=3–6 experiments. * p<0.05 compared with Am80 non-treated group.
Apoptotic cells were detected 0.2±0.2 cells and 2.1±1.6 cells in the presence and absence of 200 µM Am80 via TUNEL staining of A549 cells (Fig. 4).
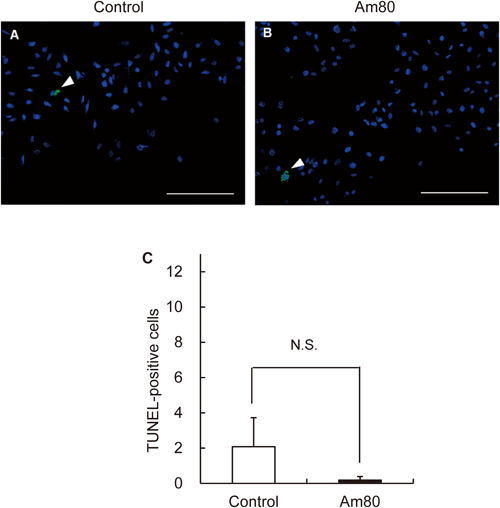
TUNEL staining in A549. A549 (625 cells/cm2) was treated with (B) or without (A) 200 µM Am80 for 24 h after 48 h pre-culture. (C) Number of TUNEL-positive cells in 500 DAPI-positive cells. Results represent the mean±S.E. of n=3 experiments. Scale bar, 200 µm.
A549 cells treated with 50 µM Am80 showed low clonogenicity. Am80 reduced the number of colonies during 2 weeks of growing in soft agar. The numbers of colonies formed per dish were approximately 41.7±11.2 and 14.7±4.7 for control and 50 µM Am80, respectively (Fig. 5).

(A) A549 (625 cells/cm2) cells were incubated with or without Am80 (50 µM) for 6 d after 24-h pre-culture. Subsequently, the cells (750 cells/cm2) were subjected to a soft agar colony formation assay. The colonies were stained with crystal violet. Results represent the mean±S.D. of n=3 experiments. * p<0.05 in comparison with control.
Therefore, Am80 reduced the viability of A549 cells more than 100 µM significantly at p<0.01 (Fig. 2), so we examined comparison of A549 with normal cell line MRC-5 dose of 100 and 200 µM of Am80. Am80 also reduced that of normal cell line MRC-5, however we did not confirm the difference of cell viability between A549 and MRC-5 (Fig. 6). Therefore, Am80 is not specific to cancer cells.

CellTiter-Glo assay in MRC-5 and A549. A549 and MRC-5 cell line (625 cells/cm2) was incubated with or without Am80 (100 and 200 µM) for 6 d after 24-h for pre-culture. Results represent the mean±S.D. of n=3 experiments. * p<0.05 A549 vs. MRC-5 cell line group for each dose. † <0.01, †† <0.01 vs. control treatment group for each dose: † is A549 and †† is MRC-5. For plots of cell survival rate, the mean basal value (Control) of each cell line group was set at 100%.
In this study, we investigated the effect of Am80 on pulmonary carcinoma cell growth. Am80 reduced the viability of A549 cells (Fig. 1) and anchorage-independent growth ability (Fig. 4). Our data showed that Am80 inhibited the growth of A549 cells. We also conducted the comparison of Am80 with ATRA. ATRA also reduced the viability of A549 (Fig. 2), although Am80 has higher potential for antiproliferative effect comparison with ATRA. It is in consistency with previous reports that Am80 is more powerful property.8,9) We demonstrated that Am80 might be effective as a novel cell-growth inhibitor in pulmonary carcinoma. Other previous experiments had shown that Am80 inhibits the growth of human breast cancer cells and the human glioblastoma cell line and stimulates or induce apoptosis.14,15) In the present study, however, as for the apoptotic cells, there were no significant differences between the two groups in the presence and absence of 200 µM Am80 within 24-h treatment (Fig. 4). So, Am80 may not induce apoptosis, or apoptosis may not be the direct cause of growth inhibition. Am80 has been reported to stimulate the differentiation of acute promyelocytic leukemia cells into neutrophils and induces neuronal differentiation in a human neuroblastoma NH-12 cell line.16,17) The antiproliferative mechanism of Am80 in pulmonary carcinoma is still unknown; anaplastic pulmonary carcinoma cells might be useful in the studies of this mechanism.
CRABP-I expression is associated with metabolism and CRABP-II expression enhanced RAR-mediated transcriptional activation of a reporter gene.12,18) We demonstrated that Am80 increased expression level of CRABP-II (Fig. 3), but CRABP-I was not induced (data not shown). CRABP-II localizes to the nucleus in response to ligand.19) We suggest that CRABP-II induced by Am80 may promote nuclear translocation of Am80, and Am80 may also adjust CRABP-II expression through a nuclear receptor. Therefore, we conducted the gene knockdown of CRABP-II, though could not be confirmed that gene-knockdown efficiency of CRABP-II was only about 40% (data not shown). Therefore, we did not examine that knockdown of CRABP-II actually cancels the inhibition of A549 growth by Am80.
Am80 is a RAR-α and RAR-β selective agonist.17) RAR-β transcription is epigenetically regulated by RAR-α, and the induction of RAR-β expression is important in tumor cell growth inhibition.20) In addition, Am80 is associated with the phosphoinositide 3-kinase/Akt signal transduction pathway during HL-60 cell-growth suppression at the late stages of differentiation.21) To elucidate the relationship between those molecular mechanisms and Am80 activity, it is important to understand the antiproliferative effect of Am80.
For the clinical application of Am80, that safety is an important issue. Comparison of A549 with normal cell lines is important when viewed from the perspective of toxicity. We conducted the comparison of them. Am80 also reduced that of normal cell line MRC-5, however we did not confirm the difference of cell viability between A549 and MRC-5 (Fig. 6). Therefore Am80 is not specific to cancer cells. From this result, we need the strategy of reducing toxicity against normal cells and targeting to cancer cells.
The effectiveness of Am80 on the growth of lung cancer tissue has not been reported. Furthermore, to assess the antiproliferative effect of Am80, we conducted in vivo experiment with mice harboring sc tumor of A549. The mice treated Am80 (1.0 mg/kg) and measured tumor volume at twice a week. In the results, we did not confirm the antiproliferative effect of Am80 till day 14 from intratumor administration of Am80 (data not shown). Further study such as additional follow-up, increasing of dose and so on is needed.
Am80 were kindly provided by Dr. Koichi Shudo (Japan Pharmaceutical Information Center). This work was partially supported by The Mochida Memorial Foundation for Medical and Pharmaceutical Research (M. Horiguchi) and the Hamaguchi Foundation for the Advancement of Biochemistry (M. Horiguchi).
The authors declare no conflict of interest.