2016 Volume 39 Issue 3 Pages 384-393
2016 Volume 39 Issue 3 Pages 384-393
We investigated the relationship between noradrenaline (NAd)-induced contractions, endothelial function, and hypertension in femoral arteries isolated from spontaneously hypertensive rats (SHR). In the femoral arteries of SHR, vs. age-matched control Wistar Kyoto (WKY) rats, contractions induced by NAd were increased. These effects were enhanced by endothelial denudation, which abolished the differences between the two groups. NAd-induced contractions were enhanced by nitric oxide (NO) synthase inhibition, and further increased by the blockade of endothelium-derived hyperpolarizing factor (EDHF). Conversely, NAd-induced contractions were inhibited by cyclooxygenase (COX) inhibition. In addition, in SHR arteries, acetylcholine-induced relaxation was reduced, and components of endothelium-derived factors were altered, such as increased COX-derived vasoconstrictor prostanoids, reduced EDHF, and preserved NO-mediated relaxation. In the femoral arteries of SHR, the production of prostanoids [6-keto prostaglandin (PG)F1α (a metabolite of prostacyclin (PGI2), PGE2, and PGF2α] and COX-2 protein were increased compared with that in WKY rats. By contrast, contractions induced by beraprost (a stable PGI2 analogue), PGE2, and U46619 (thromboxane/prostanoid receptor agonist) were similar between the SHR and WKY groups. Thus, NAd-induced femoral arterial contractions are augmented in SHR resulting from endothelial dysfunction and increased COX-derived vasoconstrictor prostanoid levels.
Hypertension is one of the most common chronic diseases in humans,1) and hypertension-associated vascular complications such as stroke, heart failure, kidney diseases and peripheral arterial diseases are major sources of morbidity and mortality that exacerbate the quality of life.1–3) Because dysfunction and abnormal signaling in the main structural elements of the vasculature, such as endothelial cells and smooth muscle cells, are characteristic hypertension-associated vascular complications,4–9) and functional impairments in these cells are observed in hypertensive patients and animal models of the disease,10–19) a comprehensive understanding of the underlying mechanisms is indispensable for preventing and treating such complications.
Among endothelium-derived factors, nitric oxide (NO) is of the greatest importance for modulating vascular function.20,21) However, other factors including endothelium-derived hyperpolarizing factor (EDHF) and cyclooxygenase (COX)-derived prostanoids can also modulate vascular tone.22–25) Indeed, there are several reports suggesting that contraction induced by various substances including alpha-adrenoceptor ligands is enhanced by the inhibition of EDHF signaling26) and enhanced27) or suppressed11,28,29) by COX signaling in various arteries under a (patho)physiological state. Because prostacyclin (PGI2) serves as an endothelium-derived vasodilator and vasoconstrictor depending on the vessel type, species, or disease condition,4,9,30) the exact role of COX-derived prostanoids to regulate vascular tone is complicated in cardiovascular diseases including hypertension.
Despite the imbalance between endothelium-derived relaxing and contracting factors in the vasculature in patients with hypertension, their exact effects on endogenous vasoconstrictor-induced contraction in femoral arteries remain unknown. In the present study, therefore, we hypothesized that femoral arteries exhibit enhanced responsiveness to noradrenaline (NAd) in the presence of hypertension due to alterations of the modulative effect of the endothelium. To test this hypothesis, we used femoral arteries isolated from spontaneously hypertensive rats (SHR) and control Wistar Kyoto (WKY) rats and distinguished the components of endothelium-derived factors to evaluate NAd-induced contraction using pharmacological approaches.
NAd, apamin, indomethacin, NG-nitro-L-arginine (L-NNA), TRAM-34 (1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole), phenylephrine (PE), and β-actin antibody were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Acetylcholine chloride (ACh) was purchased from Daiichi-Sankyo Pharmaceuticals (Tokyo, Japan). Sodium nitroprusside (SNP) was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Antibodies for COX-1 and COX-2 and valeroyl salicylate (VAS), NS398, beraprost, prostaglandin (PG)E2, U46619, and enzyme immunoassay kits for 6-keto PGF1α (a metabolite of PGI2), PGE2, PGF2α, and thromboxane (TX)B2 (a metabolite of TXA2) were procured from Cayman Chemical (Ann Arbor, MI, U.S.A.).
Animals and Measurement of Blood PressureFour-week-old male SHR and control WKY rats were obtained from Hoshino Laboratory Animals, Inc. (Ibaraki, Japan). Water and food were given ad libitum in a controlled environment (room temperature: 21–22°C, humidity: 50±5%) until the rats were 15–18 weeks old. This study was approved by the Hoshi University Animal Care and Use Committee, and all studies were conducted in accordance with the “Guide for the Care and Use of Laboratory Animals” published by the U.S. National Institutes of Health and with the “Guide for the Care and Use of Laboratory Animals” adopted by the Committee on the Care and Use of Laboratory Animals of Hoshi University (accredited by the Ministry of Education, Culture, Sports, Science and Technology of Japan). Systolic blood pressure was measured using the tail cuff method as previously described.31,32)
Vascular Functional StudiesMeasurements of the isometric force of femoral arteries and data analysis were essentially conducted as described previously.33,34) The concentration–response curves for NAd (10−10–10−4 M), PE (10−10–10−4 M), PGE2 (10−9–10−5 M) and U46619 (10−10–10−7 M) were obtained using endothelium-intact or endothelium-denuded femoral arterial rings. For the relaxation study, the concentration–response curves for ACh (10−9–10−5 M), SNP (10−10–10−5 M), and beraprost (10−9–10−5 M) were obtained using endothelium-intact rings pre-constricted with serotonin (10−7–3×10−7 M).
To investigate the effects of various inhibitors on the NAd-induced contraction or ACh-induced relaxation, a given ring was incubated for 30 min in the appropriate drug-containing medium (viz. 10−5 M indomethacin [a COX inhibitor34]]), 10−4 M L-NNA (an NO synthase (NOS) inhibitor34)), 10−5 M TRAM-34 (an IKCa inhibitor12,26)), 10−7 M apamin (an SKCa inhibitor26)), 10−4 M VAS (a selective COX-1 inhibitor34,35)), 10−6 M NS398 (a selective COX-2 inhibitor34,35)), before the cumulative addition of NAd or ACh. These drug concentrations were chosen based on previous studies. Endothelium-denuded femoral arterial rings were obtained by infusing a 0.1% 3-([3-cholamidopropyl]dimethylammonio)-1-propanesulfonate solution for 1 min, which was subsequently flushed out with modified Krebs–Henseleit solution (KHS); the inability of ACh to relax these rings confirmed the success of this procedure, as reported previously.34)
Release of ProstanoidsThe release of prostanoids was measured by enzyme immunoassay (EIA) as performed in our previous studies.34) Each femoral arterial ring was placed for 30 min in a siliconized tube containing KHS at 37°C, and then 10−5 M NAd was applied for 20 min. Next, after the femoral rings had been removed, the tubes were freeze-clamped in liquid nitrogen and stored at −80°C for subsequent analysis. 6-Keto PGF1α (a metabolite of PGI2), PGE2, PGF2α, and TXB2, a metabolite of TXA2, were measured using commercially available EIA kits (Cayman Chemical, Ann Arbor, MI, U.S.A.). The amount of prostanoid released was expressed in nanograms (6-keto PGF1α) or picograms (PGE2, PGF2α, and TXB2) per milligram wet weight of the femoral artery.
ImmunoblottingImmunoblotting and data analysis were performed as previously reported.34)
Data AnalysisData are expressed as the mean±standard error (S.E.). Contractile responses of the femoral artery are expressed as a percentage of the contractile response to 80 mM KCl, and relaxant responses are expressed as a percentage of the serotonin-induced pre-contraction (0% means maximal contraction of serotonin and 100% means baseline tension before serotonin application). For the functional studies, individual concentration–response curves were analyzed by nonlinear regression curve fitting and calculated the negative log EC50 (pD2), maximum response values (Emax), and area under the curve (AUC), using Graph Pad Prism (v. 5.0; GraphPad Software Inc., San Diego, CA, U.S.A.). Statistical evaluations were performed using Student’s t-test between two groups and one-way ANOVA followed by Bonferroni’s test or Dunnett’s test for comparisons involving or more groups. Values of p<0.05 were considered significant.
At the time of the experiment, the body weight was similar between SHR (366.1±3.1 g, n=61) and WKY rats (364.5±2.9 g, n=62), whereas systolic blood pressure was greater in SHR (184±2 mmHg, n=61, p<0.001) than in WKY rats (110±2 mmHg, n=62).
Relationships between Endothelium and NAd- and PE-Induced Contraction in the Femoral ArteryBecause NAd- or PE-induced contraction was modulated by the endothelium in various arteries,26,28,36–38) in the first series of experiments, we investigated whether contraction induced by NAd (Fig. 1A) and PE (Fig. 1B) is altered in femoral arteries from SHR and WKY rats.

Concentration–response curves for NAd (10−10–10−4 M) (A) and PE (10−10–10−4 M) (B) in endothelium intact and endothelium denuded (EC−) femoral arteries obtained from SHR and WKY. Data are presented as the mean±S.E.; n=9–13. * p<0.05, WKY vs. WKY EC−. # p<0.05, SHR vs. SHR EC−.
As shown in Fig. 1A and Table 1, NAd-induced contraction was increased in femoral arteries from SHR compared to those from WKY rats. The contraction was enhanced by endothelium denudation in both groups. It should be noted that the difference in NAd-induced contraction between SHR and WKY rats was abolished in endothelium-denuded femoral arteries.
| SHR | WKY | |||||
|---|---|---|---|---|---|---|
| Emax | pD2 | AUC | Emax | pD2 | AUC | |
| NAd-induced contraction | ||||||
| EC intact | 131.2±16.7 (12) | 5.31±0.14 (12) | 194.0±32.6 (12) | 79.8±12.9 (12) | 4.99±0.13 (10) | 87.9±22.6 (12) |
| EC− | 165.1±14.2 (9) | 5.80±0.07 (9)* | 311.8±23.4 (9)* | 147.8±16.0 (9)* | 6.19±0.05 (9)* | 329.5±36.5 (9)* |
| PE-induced contraction | ||||||
| EC intact | 94.8±12.7 (13) | 5.16±0.11 (13) | 130.4±25.1 (13) | 56.0±8.8 (12) | 4.86±0.09 (12) | 61.3±11.9 (12) |
| EC− | 181.4±20.5 (9)* | 5.72±0.13 (9)* | 312.2±38.9 (9)* | 166.6±10.7 (13)* | 5.91±0.08 (13)* | 318.7±26.3 (13)* |
Values are presented as the mean±S.E. Number of experiments is shown within parentheses. * Significant difference from the corresponding EC intact (p<0.05 by Bonferroni’s test).
Similar data were obtained for the α1 adrenoceptor agonist PE (Fig. 1B, Table 1).
ACh-Induced Endothelium-Dependent and SNP-Induced Endothelium-Independent Relaxation in the Femoral ArteryWe next investigated relaxation induced by endothelium-dependent or endothelium-independent vasodilators. As shown in Fig. 2A and Table 2, ACh-induced relaxation was reduced in femoral arteries from SHR (vs. WKY rats). On the contrary, SNP-induced relaxation was almost similar between the two groups (Fig. 2B, Table 2).

Concentration–response curves for ACh (10−9–10−5 M) (A) and SNP (10−10–10−5 M) (B) in femoral arteries pre-constricted with serotonin (10−7–3×10−7 M). Data are presented as the mean±S.E.; n=8. * p<0.05, SHR vs. WKY. n.s., not significant.
| SHR | WKY | |||||
|---|---|---|---|---|---|---|
| Emax | pD2 | AUC | Emax | pD2 | AUC | |
| ACh | 82.9±3.7 (8)* | 7.33±0.11 (8) | 187.6±14.1(8)* | 93.0±1.9 (8) | 7.52±0.10 (8) | 234.8±13.0 (8) |
| ACh (Indo/TRAM-34/Apa) | 87.8±2.5 (12) | 7.54±0.09 (12) | 215.0±11.4 (12) | 90.9±1.4 (12) | 7.42±0.08 (12) | 211.6±7.3 (12) |
| ACh (L-NNA/Indo) | 38.0±9.7 (10)* | 7.33±0.18 (10) | 69.8±18.7 (10)* | 63.8±7.3 (12) | 7.11±0.11 (12) | 130.6±19.1 (12) |
| ACh (Indo) | 92.8±1.3 (12) | 7.72±0.05 (12) | 242.7±5.9 (12) | 90.4±2.2 (12) | 7.53±0.10 (12) | 222.6±11.8 (12) |
| SNP | 96.8±1.1 (8) | 7.66±0.13 (8) | 257.4±13.0 (8) | 98.8±0.9 (8) | 7.91±0.11 (8) | 286.8±11.6 (8) |
Values are presented as the mean±S.E. Number of experiments is shown within parentheses. ACh: acetylcholine, Indo: indomethacin (10−5 M), TRAM-34: [1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole] (10−5 M), Apa: apamin (10−7 M), SNP: sodium nitroprusside. * Significant difference from the corresponding WKY (p<0.05 by Student t-test).
There are three major substances known as EDRFs including PGI2, EDHF, and NO.20,23,30,39) Moreover, COX-derived substances exert two opposite responses, namely vasodilation (i.e., PGI2) and vasoconstriction.24,25,40) To evaluate which components are altered in the SHR femoral artery, we assessed concentration–response curves for ACh in the presence of various inhibitors to block the aforementioned components (Fig. 3). Indomethacin (a non-selective COX inhibitor, 10−5 M) markedly increased ACh-induced relaxation in femoral arteries from SHR (Fig. 3A vs. Fig. 2A). Non-NO- and non-PGI2-type relaxation, namely EDHF-type relaxation, was impaired in femoral arteries from SHR (vs. WKY rats) (Fig. 3B). Conversely, ACh-induced NO-mediated relaxation under COX (indomethacin) and blockade of IKCa/SKCa, which is major source of EDHF signaling (TRAM-34 plus apamin),26) was similarly preserved in femoral arteries between SHR and WKY rats (Fig. 3C). ACh-induced relaxation was largely blocked by co-treatment with these four inhibitors (viz. L-NNA, indomethacin, TRAM-34 and apamin), whereas slight response was remained in WKY arteries (Fig. 3D).

Data were obtained in the presence of the following drugs: (A) 10−5 M indomethacin (indo), (B) 10−4 M NG-nitro-L-arginine (L-NNA) plus 10−5 M indo, (C) 10−5 M indo plus 10−5 M TRAM-34 and 10−7 M apamin (Apa). (D) 10−4 M L-NNA plus 10−5 M indo plus 10−5 M TRAM-34 and 10−7 M apamin (Apa). Relaxant responses are expressed as a percentage of the pre-contraction induced by equieffective concentration of serotonin between SHR and WKY groups (viz. 10−7 to 5×10−7 M). Data are presented as the mean±S.E.; n=12 (A), n=10–12 (B), n=12 (C), and n=5 (D). * p<0.05 vs. corresponding WKY group. n.s., not significant.
To investigate to what extent the aforementioned observed factors (viz. NO-, EDHF-, and COX-derived prostanoids) contributed to NAd-induced contraction in femoral arteries, concentration–response curves for NAd in the presence of various inhibitors were evaluated (Fig. 4). As shown in Fig. 4A and Table 3, NAd-induced contraction was enhanced by a NOS inhibitor in femoral arteries from both SHR and WKY rats. It should be noted that the enhancement of NAd-induced contraction under the condition of NOS inhibition was greater in SHR than in WKY rats (Fig. 4A, Table 3).

Data were obtained in the presence of the following drugs: (A) 10−4 M L-NNA, (B) 10−4 M L-NNA in combination with 10−5 M TRAM-34 plus 10−7 M apamin (Apa), (C) 10−5 M indomethacin (indo). Data are presented as the mean±S.E.; n=8 (A), n=10–12 (B), n=6–8 (C). * p<0.05, SHR vehicle (Veh) vs. WKY Veh. # p<0.05, L-NNA-treated SHR vs. L-NNA-treated WKY. † p<0.05, WKY Veh vs. L-NNA/TRAM-34/Apa-treated WKY group. ‡ p<0.05, Veh-treated SHR vs. indo-treated WKY.
| SHR | WKY | |||||
|---|---|---|---|---|---|---|
| Emax | pD2 | AUC | Emax | pD2 | AUC | |
| Vehicle | 139.5±16.3 (8)* | 4.90±0.15 (8) | 156.3±22.4 (8)* | 66.0±16.3 (8) | 4.50±0.41 (8) | 51.7±10.5 (8) |
| L-NNA | 150.9±15.5 (8) | 5.59±0.10 (8) | 250.9±34.8 (8)* | 97.5±14.6 (8) | 4.93±0.15 (8) | 119.3±26.6 (8) |
| Vehicle | 130.2±18.0 (10)* | 5.51±0.09 (10) | 212.5±38.0 (10)* | 53.8±10.9 (12) | 5.21±0.32 (9) | 83.6±24.8 (12) |
| L-NNA/TRAM-34/Apa | 148.0±11.8 (10) | 5.91±0.08 (10) | 288.1±24.4 (10) | 114.2±7.5 (12)# | 5.67±0.18 (12) | 198.8±17.0 (12)# |
| Vehicle | 123.0±14.0 (7) | 5.58±0.16 (7) | 206.0±32.1 (7)* | 67.6±18.0 (6) | 5.23±0.29 (5) | 81.1±24.9 (6) |
| Indo | 44.6±13.4 (8)# | 4.47±0.35 (6)# | 44.1±20.5 (8)# | 33.5±11.2 (7) | 4.66±0.17 (6) | 31.3±10.0 (7) |
Values are presented as the mean±S.E. Number of experiments is shown within parentheses. L-NNA: NG-nitro-L-arginine (10−4 M), Indo: indomethacin (10−5 M), TRAM-34: [1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole] (10−5 M), Apa: apamin (10−7 M). * p<0.05, corresponding WKY. # p<0.05, corresponding vehicle (Bonferroni’s test).
Under combined blocking of NO and IKCa/SKCa (viz. co-treatment with L-NNA, TRAM-34 and apamin), further enhancement of NAd-induced contraction was observed in femoral arteries from WKY rats but not in those from SHR. It should be noted that the difference of NAd-induced contraction under the condition was abolished (Fig. 4B, Table 3).
On the contrary, indomethacin reduced NAd-induced contraction in both SHR and WKY rats, and the difference in contraction between the groups was completely eliminated (Fig. 4C, Table 3).
Since opposite modulative effects such as augmentation and suppression for NAd-induced contraction were seen in inhibitions of IKCa/SKCa and COX, respectively, we next investigated which components are dominant role for NAd-induced contraction (Fig. 5). Under blockade of IKCa/SKCa, the NAd-induced contraction was greater in femoral arteries obtained from SHR than WKY. Inhibition of COX largely blocked such contractions in both groups, suggesting that the contributions for increased NAd-induced contraction in SHR femoral artery are greater in COX activity than in IKCa/SKCa activities.

Data are presented as the mean±S.E.; n=5. * p<0.05, TRAM-34/Apa-treated SHR group vs. TRAM-34/Apa-treated WKY group. # p<0.05, TRAM-34/Apa-treated SHR group vs. indo/TRAM-34/Apa-treated SHR group.
To investigate whether COX-1 and COX-2 contribute to the enhanced NAd-induced contraction in the SHR femoral artery, we generated concentration–response curves for NAd in the presence of the selective COX-1 inhibitor VAS and selective COX-2 inhibitor NS398 (Fig. 6, Table 4).
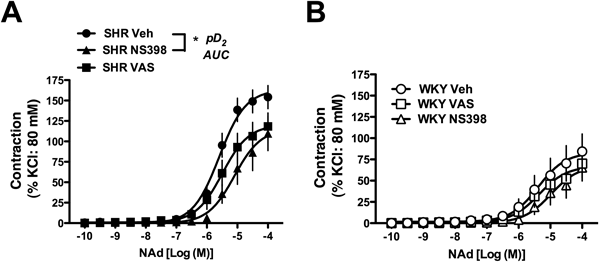
Data were obtained in the presence of the following drugs: 10−4 M valeroyl salicylate (VAS; COX-1 selective inhibitor) or 10−6 M NS398 (COX-2 selective inhibitor). The effects were evaluated in femoral arteries obtained from SHR (A) and Wistar Kyoto (WKY) rats (B). Data are presented as the mean±S.E.; n=12. * p<0.05, Veh-treated SHR vs. NS398-treated SHR.
| SHR | WKY | |||||
|---|---|---|---|---|---|---|
| Emax | pD2 | AUC | Emax | pD2 | AUC | |
| Vehicle | 154.3±14.2 (12) | 5.55±0.07 (12) | 260.1±30.7 (12) | 84.3±20.6 (12) | 5.45±0.27 (12) | 123.9±41.7 (12) |
| VAS | 118.3±16.5 (12) | 5.33±0.17 (12) | 185.3±35.3 (12) | 70.1±16.9 (12) | 5.00±0.19 (12) | 95.1±31.7 (12) |
| NS398 | 109.8±21.1 (12) | 4.49±0.35 (12)* | 128.9±32.3 (12)* | 65.5±15.9 (12) | 4.76±0.15 (12) | 70.1±23.5 (12) |
Values are presented as the mean±S.E. Number of experiments is shown within parentheses. VAS: valeroyl salicylate (COX-1 inhibitor, 10−4 M), NS398: N-[2-(cyclohexyloxy)-4-nitrophenyl]-methanesulfonamide (COX-2 inhibitor 10−6 M). * p<0.05, corresponding vehicle (Dunnett’s test).
As shown in Fig. 6A, a selective COX-1 inhibitor tended to reduce NAd-induced contraction and a selective COX-2 inhibitor greatly suppressed the contraction in the SHR femoral artery. Conversely, these inhibitors did not affect NAd-induced contraction in WKY rat arteries (Fig. 6B).
Evaluations of the Release of Prostanoids and COX Protein Expression in the Femoral ArteryNext, we investigated the amount of prostanoid released by femoral arteries upon NAd stimulation (Fig. 7). Under this condition, the release of PGI2 metabolite (6-keto PGF1α) (Fig. 7A), PGE2 (Fig. 7B), and PGF2α (Fig. 7C) was significantly increased in the femoral arteries of SHR (vs. WKY rats). The release of TXA2 metabolite (TXB2) was similar between SHR and WKY rats (Fig. 7D).

Production of a PGI2 metabolite (6-keto PGF1α) (A), PGE2 (B), PGF2α, (C) and a TXA2 metabolite (TXB2) (D) upon stimulation with noradrenaline (10−5 M for 20 min). Data are presented as the mean±S.E.; n=8. * p<0.05, SHR vs. WKY.
The protein expression of COX-1 was tended to be higher in SHR than in WKY rats (although statistical significance was not reached) (Fig. 8A), whereas protein expression of COX-2 (Fig. 8B) was significantly greater in the SHR than WKY group.

Upper: Representative blots of COXs and β-actin. Lower: Densitometric data are expressed as the mean±S.E.; n=6. n.s.; not significant. ** p<0.01, SHR vs. WKY.
Finally, we investigated whether the sensitivity of prostanoid receptors would be altered in femoral artery of SHR. To confirm that PGI2 has vasorelaxant activity in femoral arteries, we obtained concentration–response curves for beraprost, a prostacyclin receptor agonist,41) in arteries pre-constricted with serotonin. As shown in Fig. 9A, beraprost induced contraction but not relaxation in femoral arteries from both SHR and WKY rats and this response was similar between two groups. Both PGE2 (Fig. 9B) and TP agonist U46619 (Fig. 9C) led to contraction in femoral arteries and these responses were similar between SHR and WKY groups.

Data are presented as the mean±S.E.; n=5–8. n.s.; not significant.
Femoral arterial dysfunction is often observed in patients with hypertension, and elucidation of the underlying mechanisms is important to control hypertension-associated peripheral arterial complications. In the present study, we found that hyperreactivity to NAd was present in the SHR femoral artery, and this augmentation is attributable to altered modulative effects of the endothelium. In particular, we demonstrated that the contributions of NO-, EDHF-, and COX-derived prostanoids, which are known as endothelium-derived factors, to NAd-induced contraction differ in the femoral artery between SHR and WKY rats.
The principal findings made in the present study are followings (Fig. 10); the enhancement of NAd-induced contraction was seen in SHR femoral arteries. Endothelium-derived relaxing factors (i.e., NO and EDHF) could suppress the NAd-induced contraction. Although the suppressive effect of NO on NAd-induced contraction was similar between SHR and WKY, EDHF-mediated suppressive effect was defected in SHR artery. COX-derived prostanoids also affected the NAd-induced contraction, and these exerted as amplifier rather than suppressor. This additive effect of prostanoids for NAd-induced contraction resulted from increased productions of prostanoids but not increased sensitivities of their receptors.

NO is one of major EDRFs, and it has suppressive effects on contraction in various arteries.20,30) In the present study, incubation of femoral arterial rings with an NOS inhibitor greatly enhanced NAd-induced contraction, suggesting an inhibitory role for NO in the modulation of femoral arterial contraction in response to NAd. Such an inhibitory effect on alpha-adrenoceptor agonist-induced contraction has been reported for the femoral artery.10) It should be noted that NAd-induced contraction under NOS inhibition was greater in femoral arteries from SHR versus WKY rats. These results suggest that the extent of the inhibitory effect of NO on NAd-induced contraction in the femoral artery may be similar between SHR and WKY rats and that other endothelial factors play a determinant role in the augmentation of NAd-induced contraction in the SHR femoral artery. Indeed, we found that ACh-induced NO-mediated relaxation was preserved in femoral artery from SHR.
In addition to NO, EDHF plays an important role in the regulation of vascular tone.20,39,42) Alterations in K+ flux in both smooth muscle cells and endothelial cells are important in EDHF signaling; however, the precise mechanisms remain unclear because the signaling differs by vessel type, species, and disease state.20,30,39) At present, the best strategy for blocking EDHF signaling is the combined inhibition of small- and intermediate-conductance KCa channels.26,39,42) In the present study, we found that NAd-induced contraction in the presence of NOS inhibition was further amplified by additional co-treatment with inhibitors of small- and intermediate-conductance KCa channels (apamin and TRAM-34, respectively) and this effect was greater in WKY rats than in SHR. Additionally, ACh-induced EDHF-type relaxation was impaired in the SHR femoral artery. Thus, these results strongly suggest that EDHF participates in the suppression of NAd-induced contraction in femoral arteries, and this inhibitory effect is minimized in the SHR femoral artery. Indeed, this finding is supported by several reports suggesting that the blockade of EDHF signaling augmented contraction induced by alpha-adrenoceptor agonists in several arteries.26) Although the ACh-induced relaxation was abolished by above inhibitors (viz. L-NNA, indomethacin, TRAM-34 and apamin) in SHR femoral arteries, such relaxation was still remained in WKY femoral arteries. In the WKY arteries, other factors such as H2O2 and epoxyeicosatrienoic acid derived from the cytochrome P450 monooxygenase39,43) metabolites may contribute to the relaxation, however, further investigation will be required on this point.
COX-derived prostanoids affect vascular tone including relaxation and contraction. Among the prostanoids, PGI2 has vasorelaxant activity via the activation of IP receptors. Several reports suggested that expression of IP receptors and their functional activities are present in hindlimb arteries including femoral arteries.44–47) However, PGI2, also has vasocontractile activity when IP receptor signaling is impaired.4,9,22,24,25) Indeed, PGI2 induces contraction in various arteries in the presence of hypertension.4,9,22,24,25,48) To confirm the roles of prostanoids in the SHR femoral artery we performed a series of experiments focused on vascular responses using pharmacological approaches, the productions of prostanoid, and COX expression. We found that the NAd-induced contraction in femoral arteries is largely inhibited by indomethacin and that the differences of NAd-induced contraction between the two groups were completely abolished. In addition, the augmentation of NAd-induced contraction in the SHR femoral artery is largely reduced by selective COX-2 inhibitor. The protein expression of COX-2 was greater in femoral arteries from SHR compared to WKY. These results suggest that prostanoids synthesized by COXs (particularly COX-2) have modulatory effects (but not inhibitory effects) on NAd-induced contraction in the SHR femoral artery. This is supported by the fact that prostanoid production was mediated by the activation of COX-2 rather than COX-1 in human femoral endothelial cells.49) As the prostanoid productions and their responses in femoral arteries, the production of a PGI2 metabolite (6-keto PGF1α), PGE2, and PGF2α, but not a TXA2 metabolite, is increased in femoral arteries from SHR upon stimulation with NAd (vs. WKY rats). On the other hand, the contractile responses induced by beraprost, PGE2, and U46619 were all similar between SHR and WKY groups. These results suggested that alterations of the production was pivotal role for increased NAd-induced contraction in SHR rather than their receptors sensitivities in femoral arteries. Although different profiles of these prostanoids productions induced by NAd in femoral arteries remain unclear, further studies will be required on this point such as expressions and activities of their synthases.
We realize that a limitation of the present study involves in the fact that we investigated large conduit femoral arteries, which are the main arteries providing blood to the lower limbs, and these arteries may not have a primary role in peripheral resistance in hypertension. However, arterial remodeling and dysfunction of endothelial and smooth muscle cells are often present in the large conduit arteries in patients and animal models with hypertension.10,11,13,16,17,37,49,50) Therefore, investigations of this artery may provide important information regarding the development of hypertension-associated vascular complications. Future research is needed to compare vascular function between large and small (resistance size) arteries in legs in the presence of hypertension.
In conclusion, our data indicate that NAd-induced contraction in the femoral artery is associated with endothelial function. The regulation of endothelial function is of significance to treat hypertension-associated peripheral vascular complications.
We thank Y. Kimoto, H. Higa, Y. Noishiki, K. Matsubara, T. Adachi, J. Takagi, A. Suwa, S. Hotozuka, H. Sashikubi, M. Takeuchi, M. Takahashi, J. Nomoto, and M. Majima for technical assistance. This study was supported in part by JSPS KAKENHI Grant Numbers 26460107, 15K21419 and 15K07975. The authors would like to thank Enago (www.enago.jp) for the English language review.
The authors declare no conflict of interest.