抄録
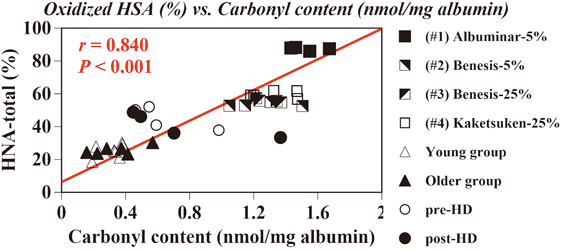
We investigated the quantitation of oxidative chemical modifications, such as thiol oxidation and carbonylation, in medical-grade human serum albumin (HSA) preparations, in comparison with those of healthy and diseased subjects. Four kinds of HSA products were obtained from three major suppliers in Japan. Eight male collegiate students and six healthy male volunteers were recruited as the young (21.6 years) and older (57.2 years) groups, respectively. Four male stable patients (64.3 years) treated with regular hemodialysis (HD) also enrolled in this study. Quantitative analyses for thiol oxidation and carbonylation were performed using HPLC and spectroscopic methods, respectively. Structural characterization was further investigated by differential scanning calorimetry (DSC) and circular dichroism (CD) spectropolarimetry. Significantly larger amounts of thiol-oxidized and carbonylated HSA products were observed than HSA obtained from healthy subjects. In the structural characterization, the midpoint temperature of the denaturation curve (Tm) analyzed by DSC was relatively high, and may have been caused by the added albumin-specific stabilizers, and CD-resolved secondary structure showed that HSA products had a helical conformation. Commercial HSA products for clinical use have a more thermally stable state and remain in a helix-rich structure, even though their specific amino acids (mainly Cys and Lys residues) are oxidatively modified.