2016 Volume 39 Issue 5 Pages 728-736
2016 Volume 39 Issue 5 Pages 728-736
Dendropanax morbifera LEVEILLE (DP) has been used in traditional Korean medicines to treat a variety of inflammatory diseases. Although the in vitro anti-inflammatory potential of this plant is understood, its in vivo efficacy and underlying molecular mechanism of anti-inflammatory effects are largely unknown. We elucidated the anti-inflammatory and analgesic activities and the underlying molecular mechanisms of DP using in vitro and in vivo models. Lipopolysaccharide (LPS)-stimulated murine macrophages were used to analyze the in vitro anti-inflammatory potential of DP extract and to elucidate the underlying mechanisms. In vivo animal models of phorbol 12-myristate 13-acetate (TPA)-induced ear edema and acetic acid-induced writhing response tests were used to analyze the in vivo anti-inflammatory effects and anti-nociceptive effects of DP extract, respectively. Methanolic extract of DP (DPME) significantly inhibited the release of nitric oxide (NO) and prostaglandin E2 (PGE2) in LPS-activated macrophages. Among the five sub-fractions, the chloroform fraction (DP-C) showed the most potent suppressive effects against pro-inflammatory mediators and cytokines in LPS-stimulated macrophages. These effects were attributed to inhibition of nuclear factor-κB (NF-κB) nuclear translocation and c-Jun N terminal kinase (JNK) 1/2 phosphorylation and to activation of NF-E2-related factor 2/heme oxygenase-1 (Nrf2/HO-1) signaling. DP-C exhibited strong protective in vivo effects in TPA-induced ear edema mouse model and acetic acid-induced writhing response test. Our data suggest that DP-C has potent anti-inflammatory and analgesic activities and may be a promising treatment against a variety of inflammatory diseases.
The quest for naturally occurring novel pharmacological agents for treating chronic pathological condition including inflammation is ongoing. Physiologically, inflammation plays important roles in maintaining homeostasis including tissue repair and remodeling.1,2) However, in chronic conditions, inflammation can lead to various diseases including inflammatory disorders, autoimmune diseases, inflammatory bowel disease, arthritis, lupus erythmatosus, endotoxin-induced organ failure, and malignancy.3–5) Control of excessive inflammation has gained a continuous attention, but the currently available drugs for treating chronic inflammatory conditions produce serious side effects; therefore, finding equally effective but less toxic agents is a current challenge in scientific research. During the process of inflammation, a wide variety of pro-inflammatory cytokines including, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-12 and interferon (IFN)-γ, are released and these cytokines up regulate the production of reactive nitrogen species and prostaglandins via the activity of inducible nitric oxide synthase (iNOS) and cyclooxygenase-II (COX-II), respectively.6,7) The excessive release of these inflammatory mediators is involved in several inflammatory diseases; therefore, these serve as potential targets for development of new anti-inflammatory agents.
The induction of the cytokines and responsible enzymes for inflammatory mediators can be modulated by several intracellular signaling. Nuclear factor-κB (NF-κB) is a transcription factor that plays a crucial role in inflammation through regulation of pro-inflammatory cytokines.8) In its inactive form, NF-κB is located in the cytoplasm in association with endogenous inhibitory protein IκB (inhibitor of NF-κB). However, in response to stimuli such as lipopolysaccharide (LPS), an endotoxin from Gram-negative bacteria, IκB kinase (IKK) mediates the phosphorylation and degradation of IκB, resulting in NF-κB activation and its subsequent translocation to the nucleus, where it binds to the promoter and regulates the expression of a variety of inflammatory genes.9,10) Mitogen activated protein kinases (MAPKs) are a group of serine/threonine protein kinases responsible for integrating and processing various signals from the extracellular environment to the inside of the cell and comprise three distinct pathways: extracellular signal-regulated kinase (ERK), p38, and c-Jun N-terminal kinase (JNK).11) In response to stimuli such as microbial infection, MAPKs become activated and positively regulate inflammatory mediators such as IL-6, IL-1β, TNF-α, iNOS, and COX-II,12) leading to inflammation.
NF-E2-related factor 2 (Nrf2) is a transcription factor pertaining to basic leucine zipper genes.13) Under normal physiological conditions, Nrf2 is bound to Kelch-like ECH-associated protein 1 (Keap1), a negative regulator, in the cytoplasm. However, in response to stress, Nrf2 dissociates from its repressor Keap1 and translocate to the nucleus, where it binds to anti-oxidant response element (ARE) to mediate transcription of several anti-oxidant genes including heme oxygenase-1 (HO-1).14) HO-1 induction via increased Nrf2 nuclear translocation has been shown to contribute to attenuation of inflammatory reaction.15) These intracellular signaling pathways have been suggested as potential targets to modulate inflammatory reaction.
Dendropanax morbifera LEVEILLE (DP), a subtropical tree called Hwangchil in Korea due to its golden color lacquer, belongs to the Araliaceae family. It is an endemic species in Korea and is found in the southwestern parts of the country.16) Various parts including stems, roots, leaves, and seeds of this plant have been used in traditional Korean medicines for treating diseases including migraine, skin and infectious diseases, and dysmenorrhea.17) Methanolic extracts of debarked stems and leaves have been shown to possess anti-oxidant and anti-cancer properties.18) Several studies have demonstrated that various parts of DP exhibit hepatoprotective,19) anti-oxidant,20) anticomplement,21,22) anti-diabetic,23) anti-atherogenic,24) larvicidal,25) and anti-plasmodial effects.26) A cycloartane-type triterpene glycoside, oleifolioside, has also been isolated from stems of DP and found to possess anti-inflammatory activity in vitro.27) We have recently proved the protective effects of the chloroform fraction of this plant against cisplatin-induced nephrotoxicity.28) In a recent study, the green and senescent leaves of this plant have been demonstrated to possess anti-inflammatory activity in an in vitro model.29) Despite the emerging evidence for biological benefits of DP, the analgesic and anti-inflammatory activities of this plant and the underlying molecular mechanisms of its extracts have not been studied in detail.
In this study, we have demonstrated the mechanism underlying anti-inflammatory activity of DP, along with its in vivo relevancy. We investigated the anti-inflammatory potential of total methanol extracts of DP and its sub-fraction. Among the five fractions, the chloroform fraction (DP-C) was found to be the most potent without affecting the number of viable cells. DP-C showed potent in vitro and in vivo anti-inflammatory activities and also exhibited anti-nociceptive effects in the acetic acid-induced writhing test of an animal model. The anti-inflammatory activity of DP-C was attributed to the inhibition of NF-κB nuclear translocation and JNK1/2 phosphorylation and activation of Nrf2-HO-1 signaling. We suggest that DP-C may provide an important clue to develop anti-inflammatory drug candidate.
Dimethyl sulfoxide (DMSO), lipopolysaccharide (LPS, Escherichia coli O111:B4), sodium nitrite, indomethacin, 2-mercaptoethanol, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and arachidonic acid were obtained from Sigma-Aldrich (St. Louis, MO, U.S.A.). Primary antibodies against iNOS, COX-II, and Nrf2 were acquired from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, U.S.A.). HO-1 was obtained from Enzo Life Sciences (Plymouth Meeting, PA, U.S.A.); glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from Millipore (Billerica, MA, U.S.A.); and NF-κB P65, p-p44/42 MAPK (Thr202/Tyr204), p44/42 MAPK, p-p38 MAPK (Thr180/Tyr182), p38 MAPK, p-stress-activated kinase (SAPK)/JNK (Thr183/Tyr185), SAPK/JNK, Lamin A/C were purchased from Cell Signaling Technology (Beverly, MA, U.S.A.). Goat anti-mouse immunoglobulin G (IgG) horseradish peroxidase (HRP)-conjugated secondary antibody was obtained from Santa Cruz Biotechnology Inc., and goat anti-rabbit IgG HRP conjugated secondary antibody from Biorad (Hercules, CA, U.S.A.).
Plant Material and Preparation of ExtractsPreparation of plant material was performed as previously described.28) Leaves of DP were purchased at a local herbal market in the southern area of Korea. The plant was authenticated, and a voucher specimen was deposited in the Herbarium of College of Pharmacy, Hanyang University (No. HYUP-DP-001). The dried leaves of DP (1 kg) were extracted with 3 L of 70% MeOH for two weeks at room temperature. The extract solution of DP was vaporized under reduced pressure to yield 167 g of crude total methanolic extract (yield 16.7%). For fractionation, the methanolic extract of DP was successively partitioned with n-hexane (28.0%), chloroform (2.5%), ethyl acetate (19.0%), n-butanol (39.6%) and remnant H2O layer (7.8%). Each extract was vaporized to dryness under reduced pressure and stored at 4°C. The concentrations of DP extracts used in our in vitro and in vivo studies were determined based on our pilot studies.
Cell CultureRAW264.7 cells were acquired from ATC C (VA, U.S.A.). The cells were maintained at 37°C in a humidified atmosphere containing 5% CO2 and 95% air in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, NY, U.S.A.) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco), 100 units/mL of penicillin, and 100 µg/mL of streptomycin (Welgene, South Korea). Experiments were performed on cells at 80% confluence. Macrophages were treated with LPS to induce inflammatory reactions.30) Each dried extract was completely dissolved in absolute DMSO, and then were diluted with culture medium (final 0.1% DMSO) to treat the cells.
Cell Viability DeterminationTo determine the effect of methanolic extract of DP (DPME) or DP fractions on RAW264.7 cell viability, an MTT assay was performed. Cells were seeded in a 96-well plate at a density of 1×104 cells per well. After pre-incubation, cells were treated with the indicated concentrations of DPME or the DP fractions for 24 h. MTT reagent (10 µL; final 0.5 mg/mL) was added to each well, and the cells were further incubated for 2 h at 37°C. The cell-free supernatant medium was removed, and 100 µL of DMSO was added to each well to dissolve the insoluble formazen crystals. Absorbance was quantified at 570 nm using an EnSpire multimode spectrophotometer (PerkinElmer, Inc.).
Measurement of Nitrite, Prostaglandin E2 (PGE2) and Cytokine ProductionTo determine the levels of the nitrite, PGE2 and cytokine (IL-1β, IL-6, TNF-α) in the culture medium, RAW264.7 cells were seeded in 24-well plates at a density of 1×105 cells per well and incubated at 37°C for 24 h. Cells were stimulated with LPS (200 ng/mL) for 24 h in the presence or absence of the indicated concentrations of DPME or DP fractions. The supernatant media were collected and briefly centrifuged to remove debris.
To determine nitrite level, equal volumes of conditioned media and Modified Griess reagent (Sigma-Aldrich) were mixed in a 96-well plate and incubated for 15 min at room temperature. The absorbance was quantified at 540 nm using an EnSpire multimode spectrophotometer, and a standard curve calibrated with sodium nitrite was used to calculate nitrite levels. For measurement of PGE2 and cytokine levels, enzyme immunoassay kits (R&D Systems, Minneapolis, MN, U.S.A.) were used according to the manufacturer’s instructions.
In experiment to determine the effect of DP-C on iNOS- or COX-II-mediated enzymatic reaction, we used pre-stimulated macrophages where these inducible enzymes were fully expressed, as described previously.31,32) For iNOS enzymatic reaction, the cells were stimulated with LPS for 12 h followed by two washes with phosphate buffered saline (PBS). These pre-stimulated cells were incubated in the presence or absence of DP-C for a further 12 h without LPS. The supernatant culture medium was collected, and a Griess reaction was performed to measure the level of nitrite. For COX-II enzymatic reaction, LPS was incubated for 6 h, and the cells were washed two times with fresh medium. These pre-stimulated cells were treated with the indicated concentrations of DP-C without LPS for 30 min, followed by incubation with 100 µM of arachidonic acid for 15 min. The supernatants were collected, and PGE2 concentration was measured as described above.
Western Blot AnalysisRadio immunoprecipitation assay (RIPA) buffer (Thermo Scientific, Rockford, IL, U.S.A.) with protease/phosphatase inhibitor cocktail (Thermo Scientific) was added to the RAW264.7 cells to obtain total cell lysates. The lysates were spun at 15000×g for 15 min at 4°C to remove cell debris. The concentrations of extracted proteins in the supernatants were determined using a BCA protein assay kit (Thermo Scientific). Equal amounts of protein samples were electrophoresed on a sodium dodecyl sulfate (SDS)-polyacrylamide gel and then transferred to a polyvinylidene difluoride (PVDF) membrane (BioRad, Hercules, CA, U.S.A.). Nonspecific binding was blocked with 5% skim milk in Tris-buffered saline with Tween 20 (TBST) and blotted with specific primary antibodies at 4°C overnight. The membranes were washed three times with TBST and incubated with corresponding horseradish peroxidase-conjugated secondary antibodies for 1 h. After three washes with TBST, the protein bands were examined using SuperSignal West Pico Chemiluminescent or SuperSignal West Femto maximum sensitivity substrates (Thermo Scientific). The relative band densities compared to the control were quantified using ImageJ software.
To determine NF-κB and Nrf2 nuclear translocation, the cytoplasmic and nuclear proteins were fractionated using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific) according to the manufacturer’s instructions. Lamin A/C and GAPDH were used as internal controls for nuclear and cytoplasmic extracts, respectively.
Real-Time Polymerase Chain Reaction (PCR) AnalysisCells were challenged with LPS in the presence or absence of DP-C for 6 h, followed by RNA extraction using Trizol reagent (Life Technologies, Carlsbad, CA, U.S.A.). Purified RNA (500 ng) was reverse transcribed to cDNA at 37°C for 15 min, 50°C for 5 min, and 98°C for 5 min using the ReverTra Ace® qPCR RT Kit (Toyobo, Osaka, Japan) in a MyCycler™ Thermal Cycler (BioRad). Quantitative PCR was carried out using iQTM SYBR Green supermix (Bio-Rad) on a CFX96TM Real-Time PCR Detection System (Bio-Rad), under the conditions of 95°C for 10 min, followed by 45 cycles at 98°C for 10 s, 60°C for 30 s, and 72°C for 1 min, and elongation at 72°C for 5 min. The primers were obtained from GENOTECH (Daejeon, Korea), and the sequences were as follows: for iNOS, sense 5′-CCT GGT ACG GGC ATT GCT-3′ and anti-sense 5′-GCT CAT GCG GCC TCC TT-3′; for COX-II, sense 5′-CCG TGG GGA ATG TAT GAG CA-3′ and anti-sense 5′-CCA GGT CCT CGC TTA TGA TCT G-3′; for IL-1β, sense 5′-CAA CCA ACA AGT GAT ATT CTC CAT G-3′ and anti-sense 5′-GAT CCA CAC TCT CCA GCT GCA-3′; for IL-6, sense 5′-AAC GAT GAT GCA CTT GCA GA-3′ and anti-sense 5′-GAG CAT TGG AAA TTG GGG TA-3′; for TNF-α, 5′-ATG AGC ACA GAA AGC ATG ATC-3′ and anti-sense 5′-TAC AGG CTT GTC ACT CGA ATT-3′; for GAPDH, sense 5′-TGA ACG GGA AGC TCA CTG G-3′ and anti-sense 5′-TCC ACC ACC CTG TTG CTG TA-3′. Gene expression was normalized with GAPDH, and relative mRNA expressions were calculated using the comparative CT method (2−△△Ct).
Phorbol 12-Myristate 13-Acetate (TPA)-Induced Ear EdemaThe animal protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at Hanyang University. To identify the in vivo anti-inflammatory activity of DP-C, a TPA-induced ear edema test was performed according to a previously described method.33) Male ICR mice (6 weeks old) were obtained from KOATECH (Gyeonggi, Korea), randomly divided into five groups, and housed under standard laboratory conditions of 25°C in a 12 h light/dark cycle. The mice were pre-treated with 3 mg of DP-C or 0.5 mg of indomethacin or an equal volume of vehicle by topical application on the right ear 30 min before TPA (2 µg) application. The left ear did not receive any treatment and was used as a corresponding internal control to compare thickness or weight. At 0, 1, 2, 3, and 4 h after TPA application, the thickness of each ear was measured using a digital caliper (Blue Bird, Korea), which measured the thickness to an accuracy of 0.01 mm, and the level of edema was determined by calculating the thickness difference between the right and left ears. Ear biopsies (6 mm in diameter) were collected at four hours post-TPA application, and the differences in weight between the right and left ear biopsies were considered as edema weight. All biopsies were examined after hematoxyline–eosin (H&E) staining.
Acetic Acid-Induced Writhing Response TestWe used a slightly modified method of Hiramatsu et al.34) to analyze the anti-nociceptive activity of DP-C. Mice were pre-treated with vehicle, two doses of DP-C (10 mg/kg or 50 mg/kg), or the positive control indomethacin (10 mg/kg) 30 min prior to intraperitoneal administration of 1% (v/v) acetic acid solution (10 mL/kg), a chemical irritant to induce nociception. After 5 min, the number of writhes was counted for next 10 min. Abdominal contraction with simultaneous stretching of at least one hind limb was considered as a writhe.
Statistical AnalysisThe results are presented as the mean±standard error of the mean (±S.E.M.) for all treatment groups. In vitro data were subjected to paired t-tests, and in vivo data were subjected to one-way ANOVA with post-hoc Tukey’s test in order to determine the statistical significant differences from the control using SPSS software (Chicago, IL, U.S.A.). For all analyses, a difference at p<0.05 was considered significant.
To identify the anti-inflammatory activities of DPME and its fractions, an LPS-induced macrophage system was employed. DPME significantly and dose-dependently reduced the production of NO and PGE2 in LPS-induced RAW264.7 macrophages without affecting cell viability (Figs. 1A to C). We fractionated the DPME into five fractions: Hexane, chloroform, ethyl acetate, n-butanol, and water (Fig. 1D). The effects of these fractions on anti-inflammatory mediators were investigated to determine the most active fraction. Among them, the hexane and chloroform fractions were the most active in reducing nitrite and PGE2 production (Figs. 1E, F), and cell viability data showed that the hexane fraction significantly reduced the number of viable cells (Fig. 1G). DP-C was selected as the most potent without cytotoxicity, and used for further experiments.

The effects of DPME on nitric oxide (A) and PGE2 (B) generation were examined using the Griess reaction and ELISA, respectively, in LPS-stimulated RAW264.7 cells after 24 h. (C) The effect of DPME on cell viability was determined using MTT assay. (D) Representative scheme has been shown for preparation of the fractions from total extract, and their yields. The activity of the DP fractions against LPS-induced nitric oxide (E) and PGE2 synthesis (F) in RAW264.7 cells were investigated. (G) The effect of DP fractions on cell viability was analyzed. Values are expressed as the mean±S.E.M. Significance compared with LPS-treated cells: * p<0.05; ** p<0.01; Significance compared with vehicle-treated control cells: ## p<0.01, n=03.
The most active fraction, DP-C, was further tested for its effects on inflammatory mediators in LPS-induced RAW264.7 cells. Our data showed that LPS-induced NO and PGE2 production was significantly and dose-dependently inhibited by DP-C, with IC50 values of 8.2 and 14.8 µg/mL, respectively (Figs. 2A, B). The suppressive effects of DP-C against these mediators might result from either attenuation of enzyme induction or inhibition of enzymatic reaction. We investigated the effect of DP-C against iNOS or COX-II enzymatic reactions in pre-stimulated macrophages (Figs. 2C, D). Our result showed that DP-C failed to inhibit the iNOS and COX-II enzymatic reactions, suggesting that the inhibiting effect of DP-C against inflammatory mediators may attribute to inhibit the expression of these enzymes.
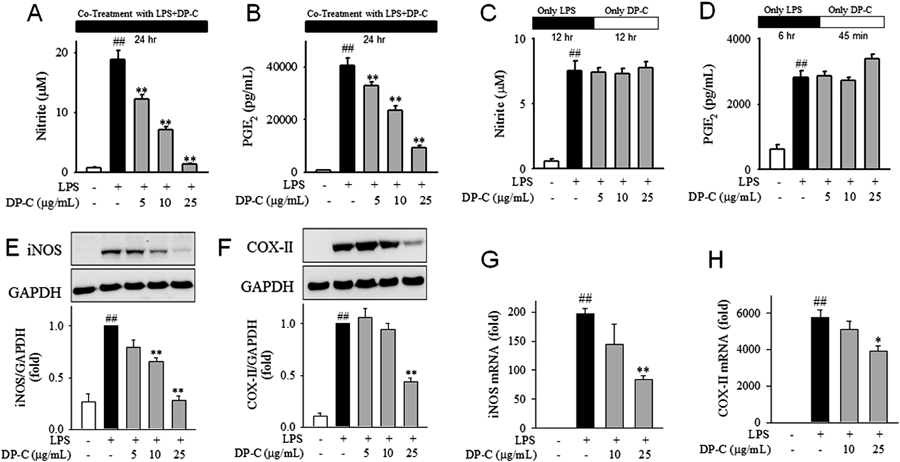
The effects of various concentrations of DP-C on LPS-induced nitric oxide (A) and PGE2 (B) levels in murine macrophages were investigated. The effect of DP-C on iNOS (C) and COX-II (D) enzyme activities was determined in LPS pre-stimulated murine macrophages after 12 h and 6 h, respectively. The effect of DP-C on iNOS (E), COX-II (F) protein expression, and iNOS (G), COX-II (H) mRNA level were determined in LPS-stimulated RAW264.7 macrophages after 24 h and 6 h, respectively. Representative images for Western blot were shown. Values are expressed as the mean±S.E.M. Significance compared with LPS-treated cells: * p<0.05; ** p<0.01; Significance compared with vehicle-treated control cells: ## p<0.01, n=03.
To evaluate the effect of DP-C on enzyme induction of iNOS and COX-II, we stimulated RAW264.7 cells with LPS in the presence or absence of DP-C. We found that DP-C significantly inhibited iNOS and COX-II protein expression in a dose-dependent manner (Figs. 2E, F). Consistent with these results, LPS-induced iNOS and COX-II mRNA expression was also reversed by DP-C (Figs. 2G, H).
Inhibitory Effect of DP-C against LPS-Induced Expression of Inflammatory CytokinesAlong with the inflammatory enzymes of iNOS and COX-II, the expression of cytokines such as interleukins and TNF-α is significantly increased to amplify the pro-inflammatory reaction. We investigated if DP-C modulates the level of inflammatory cytokines. Secreted level of IL-1β, IL-6 and TNF-α from LPS-activated macrophages were significantly reduced by DP-C (Figs. 3A to C). The level of mRNA of each cytokine was inhibited by DP-C, suggesting that these cytokines are regulated at the gene expression level (Figs. 3D to F). Cell viability data showed that the inhibitory activity of DP-C against inflammatory mediators and cytokines was not due to cytotoxicity (Fig. 3G).

The effect of DP-C on IL-1β (A), IL-6 (B), and TNF-α (C) release were determined in LPS-stimulated RAW264.7 macrophages after 24 h using ELISA kit. The effect of DP-C on mRNA expression of IL-1β (D), IL-6 (E), and TNF-α (F) were analyzed in LPS-stimulated murine macrophages after 6 h. (G) The effect of DP-C on cell viability of RAW264.7 macrophages was determined. Values are expressed as the mean±S.E.M. Significance compared with LPS-treated cells: * p<0.05; ** p<0.01; Significance compared with vehicle-treated cells: ## p<0.01, n=03.
To evaluate the underlying molecular mechanism of the anti-inflammatory effects of DP-C, we evaluated whether DP-C could modulate NF-κB and MAPKs signaling, the representative pathways of inflammation (Figs. 4A to D). We found that DP-C significantly inhibited LPS-induced NF-κB nuclear translocation and JNK 1/2 phosphorylation. However, neither LPS-induced ERK 1/2 nor p38 phosphorylation was affected by DP-C. Since HO-1 induction via Nrf2 nuclear translocation has also been suggested to produce anti-inflammatory effects.15) We determined whether DP-C also affected Nrf2-HO-1 signaling. DP-C significantly induced Nrf2 nuclear translocation and thereby induced HO-1 expression (Figs. 4E, F), suggesting that DP-C produces its anti-inflammatory effects by affecting multiple signals including NF-κB, JNK 1/2, and Nrf2-HO-1.

The effect of DP-C on NF-κB nuclear translocation (A) and MAPKs (B–D) phosphorylation was analyzed in LPS-activated murine macrophages. The effect of DP-C on Nrf2 nuclear translocation (E) and HO-1 induction (F) was determined. Lamin A/C and GAPDH were used as internal controls for the nuclear and cytoplasmic fractions, respectively. Values are expressed as the mean±S.E.M. Significance compared with LPS-treated cells: * p<0.05, ** p<0.01. Significance compared with vehicle-treated cells: # p<0.05, ## p<0.01; n=03.
To investigate in vivo anti-inflammatory activity, DP-C was topically applied to the right ear 30 min prior to edema induction by TPA. We observed a protective effect against increase in ear thickness induced by TPA at all time points examined (Fig. 5A). The increase in weight of ear plugs induced by TPA 4 h after application was also protected by DP-C (Fig. 5B). At 4 h after TPA application, cross-sections of ear discs were stained with hematoxylin and eosin for histopathological examination. TPA induced inflammatory changes such as ear edema, epidermal hyperproliferation, and increased number of neutrophils (Fig. 5C); these effects were reversed by DP-C. The extent of the anti-inflammatory action of DP-C was comparable to that of indomethacin (positive control).

DP-C (3 mg in 20 µL ethanol) or INDO (indomethacin; 0.5 mg in 20 µL ethanol) was applied topically to the right ear 30 min before TPA (phorbol 12-myristate 13-acetate; 2 µg in 20 µL of ethanol) application. Edema volume (A) at the indicated time intervals and edema weight (B) at 4 h of TPA application were measured. (C) The ear discs were stained with H&E for histological examination. n=04. (D) DP-C (10 mg/kg or 50 mg/kg) was administered intraperitoneally 30 min prior to acetic acid injection (1% solution). After 5 min, the number of writhes was counted for the next 10 min. n=05. Values are expressed as the mean±S.E.M. Significance compared with rats in stimulated groups with TPA or AA: * p<0.05, ** p<0.01. Significance compared with control rats with vehicle treatment: # p<0.05.
To observe the in vivo analgesic effect of DP-C, we employed acetic acid-induced writhing response tests in mice where inflammatory mediators play crucial roles in pain sensation.35,36) Intraperitoneal administration of acetic acid solution (1%; 10 mL/kg) induced substantial writhes in control mice pre-treated with vehicle (Fig. 5D). However, DP-C dose-dependently reduced acetic acid-induced writhes. The extent of the anti-nociceptive effect produced by DP-C was similar to that of indomethacin, suggesting that DP-C possesses potent in vivo anti-inflammatory and anti-nociceptive effects.
The present study demonstrated that the chloroform extract of DP possesses potent inhibitory activity against inflammation both in an in vitro LPS-induced macrophage model and an in vivo mouse model and has an anti-nociceptive effect in the in vivo mouse model. The underlying molecular mechanism of the anti-inflammatory effect was also elucidated. Our findings provide new insights into the mechanisms underlying the anti-inflammatory effects of DP-C and its detailed anti-inflammatory effects using in vitro and in vivo models.
Many natural resources are being used for development of new drugs, and several of the natural product-derived anti-inflammatory compounds are in different stages of development and clinical trials.15) We wanted to search for new natural products with anti-inflammatory activities that could be used for novel compounds. We made efforts to detail the anti-inflammatory and anti-nociceptive activities of Dendropanax morbifera and its underlying molecular mechanism. Although there are previous reports regarding the anti-inflammatory activity of DP, the details of underlying molecular mechanisms and its effects in vivo models are lacking.
Various phenolic compounds including rutin, quercetin, ferulic acid, naringin and naringenin were isolated from leaves of DP.29) These phenolic compounds may contribute to the anti-inflammatory activity of DP since the previous reports had proved the anti-inflammatory of these phenolic compounds.37–39) In addition, Yu et al. showed that a triterpenoid compound, oleifolioside A, isolated from lower stem parts of DP, possessed potent in vitro anti-inflammatory activity.27) Interestingly, we observed the most significant and potent anti-inflammatory activity in chloroform subfraction of DP methanolic extract. It is not likely that the previously suggested bioactive components such as phenolic compound or oleifolioside A exist in DP-C. Most of phenolic compounds identified in DP are polar in nature and they tend to be fractionated in polar solvents such as ethyl acetate, butanol and water.40) The HPLC chromatogram of DP-C revealed the existence of several minor compounds of extract, which have peak signals of different retention time as compared to retention time of major compounds in chromatogram of total extract (Supplementary Fig. 1). Further isolation and identification of bioactive compound in DP-C would be required.
Many diseases are associated with increased production of nitric oxide and PGE2 including osteoarthritis, diabetes, inflammatory bowel disease, and cancer.41–44) In the present study, we demonstrated that total DP extract and its chloroform fraction significantly inhibited LPS-induced NO and PGE2 production in a dose-dependent manner without decreasing the number of viable cells (Fig. 1). This suggests that DP is a valuable source of potential anti-inflammatory compounds.
Since the chloroform extract of DP demonstrated the highest anti-inflammatory activity among the five fractions without producing cytotoxicity, it was selected for further experiments. Using a range of concentrations, we demonstrated that DP-C significantly and dose-dependently inhibited nitrite and PGE2 levels in LPS-stimulated RAW264.7 macrophages (Figs. 2A, B) by suppressing iNOS and COX-II expression at the transcription level (Figs. 2E, F). Several cytokines including IL-1β, IL-6, and TNF-α play a key role in the pathogenesis of inflammation and pain.45,46) We have demonstrated that both mRNA expression and the secreted protein level of these cytokines are significantly inhibited by DP-C (Figs. 3A to F), suggesting its potential role in alleviating diseases associated with pain and inflammation.
NF-κB and MAPKs play critical roles in the pathogenesis of inflammation and inflammatory diseases.8,12) We demonstrated that DP-C significantly inhibited NF-κB nuclear translocation and JNK 1/2 phosphorylation (Figs. 4A, B). Since DP-C did not inhibit LPS-induced ERK 1/2 phosphorylation, as shown by Western blotting (Fig. 4C), our results do not support the inhibition of ERK1/2 phosphorylation demonstrated by Hyun et al.29) The role of HO-1 induction via Nrf2 nuclear translocation has been demonstrated to possess beneficial effects in the diseases associated with inflammation.47) In our study, we found that DP-C induced HO-1 expression via Nrf2 nuclear translocation (Figs. 4E, F). Inhibition of NF-κB nuclear translocation and JNK 1/2 phosphorylation and induction of Nrf2-HO-1 signaling are the key underlying mechanisms of the anti-inflammatory effects produced by DP-C.
To prove the in vivo relevancy of anti-inflammatory activity of DP-C, we have applied two different in vivo models. TPA-induced ear edema mouse model is well-established in vivo inflammation model and it has been widely used for pharmacological and toxicological studies. In this model, the inflammation is induced by complex processes including activation of intracellular pathways such as MAPKs and NF-κB, the release of inflammatory mediators and cytokines, and the infiltration of neutrophils and macrophages.48,49) Of note, inflammatory reactions both in keratinocytes and in infiltrated immune cells including macrophages play important roles in induction of ear edema. We examined if DP-C modulates inflammatory reaction in keratinocytes stimulated by combination of IFN-γ and TNF-α, but DP-C failed to show inhibitory effect of NO release in keratinocyte with the same concentration tested in the macrophages (Supplementary Fig. 2). It seems that inflammatory reactions in macrophages are more susceptible to inhibitory effects of DP-C than in keratinocytes, and this might play major role in the attenuation of TPA-induced ear edema (Figs. 5A to C).
In addition to the ear edema, we have tested the analgesic effect of DP-C in acetic acid-induced writhing test. Pain is one of the cardinal signs of the inflammation caused by the action of inflammatory mediators on peripheral sensory neurons.35) Acetic acid-induced writhing test is commonly employed to investigate the peripheral analgesic effects of drugs. Intraperitoneal administration of acetic acid causes tissue damage resulting in the release of prostaglandins that activate pain receptors on sensory neurons.36) The release of pro-inflammatory cytokines by resident peritoneal macrophages is also responsible for nociception in this model,50) suggesting that prostaglandins and cytokines are the important targets to treat pain. DP-C showed potent anti-nociceptive activity in acetic acid-induced writhing test (Fig. 5D), and this result matches well to our in vitro observation on inhibitory effect of DP-C against release of PGE2 and cytokines.
In summary, we demonstrated that DP-C ameliorated LPS-induced release of inflammatory mediators including NO and PGE2 in murine macrophages by suppressing iNOS and COX-II expression at the gene level. Up-regulation of pro-inflammatory cytokines including IL-1β, IL-6 and TNF-α were also suppressed by DP-C. These anti-inflammatory effects are mediated by the inhibition of NF-κB nuclear translocation and JNK 1/2 phosphorylation as well as the induction of HO-1 via Nrf2 signaling. We also demonstrated the in vivo relevance of the anti-inflammatory and anti-nociceptive activities of DP-C using mouse models. Our findings suggest DP-C as a valuable source for anti-inflammatory compounds.
This work was supported by a Grant from Hanyang University (201400000001165).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.