2016 Volume 39 Issue 5 Pages 737-746
2016 Volume 39 Issue 5 Pages 737-746
Doxorubicin (DOX) is one of the best known anticancer drugs, and is used in the treatment of lymphoma, lung cancer, stomach cancer, and a number of other cancers. However, DOX has some serious side effects, the worst being lethal heart failure. Occasionally, its side effects result in the cessation of the anticancer treatment, thus having a serious adverse influence on prognosis. Agents that can be administered as alternative prophylactics or to ameliorate the side effects of DOX will be useful in increasing the safety and efficacy of anticancer therapy. Adrenomedullin (AM) is a peptide hormone secreted by many organs, including the heart; it has an organ-protective effect, including antiapoptotic, anti-inflammatory, and antioxidative stress. Blood AM levels increase with heart failure; endogenic AM has been suggested in order to protect the heart. Furthermore, exogenous AM administration has shown therapeutic effects for heart failure in patients. However, it is unclear whether AM can protect the heart against drug-induced cardiac injury in vivo. The present study was performed in order to investigate the effects of AM on DOX-induced cardiac damage. Male BALB/c mice were treated with DOX and/or AM. Exogenous AM improved the survival ratio of DOX-treated mice. In addition, AM reduced serum lactate dehydrogenase (LDH) levels following DOX treatment. On pathological examination, AM was shown to inhibit DOX-induced cardiac tissue damage, mitochondrial abnormality, and cell death. These findings suggest that AM has a protective effect against DOX-induced cardiac damage.
Doxorubicin (DOX) is an anthracycline drug that was discovered in 19691) and is used in the treatment of several cancers, including lymphoma, myeloma, breast, ovarian, lung, gastric, thyroid, sarcoma, and pediatric cancers.2,3) Serious side effects of drugs frequently have a negative effect on the QOL of patients undergoing anticancer treatment. DOX-induced lethal heart failure4) has been reported to interfere with treatment.5) DOX induces free radical production6) and mitochondrial disruption in cardiac myocytes.7) Free radicals, including reactive oxygen species (ROS) and DOX metabolite radicals, damage DNA and cell membranes. In addition, DOX has been shown to combine with the mitochondrial inner membrane and inhibit electron transport.8,9)
First discovered in 1993,10) adrenomedullin (AM) is a peptide hormone secreted by many organs and cells, including the heart,11) which acts as a local autocrine/paracrine mediator.12) AM combines with a 7-transmembrane domain G protein-coupled receptor on the cell membrane called calcitonin receptor-like receptor (CLR),13) and acts as a bioactive peptide. The levels of endogenous AM in serum and cardiac tissue are increased in hypertension,14,15) heart failure,16) and acute myocardial infarction.17) In addition, AM has several functions, including antioxidative,18,19) anti-inflammatory,20) and angiogenic effects,21) as well as acting as a regulator of mitochondrial function.22)
In AM gene knockout (AM KO) mice, homozygotes died in utero with abnormal cardiovascular development, and heterozygotes showed elevated blood pressure.23) Cardiac hypertrophy and fibrosis were observed in AM KO heterozygotes when subjected to cardiovascular stress.24,25)
Exogenous AM administration resulted in reduction of inflammation26,27) and enhancement of wound healing28) in mouse models. In human acute heart failure, AM enhances recuperation from cardiac damage.29) These observations suggest that AM is a cardioprotective agent.
A recent study indicated that AM reduced DOX-induced cardiac myocyte cell death in vitro.30) However, it is not yet clear whether exogenous AM administration can protect the heart against DOX-induced cardiac damage in vivo.
Male BALB/c mice (9 weeks old) were purchased from CLEA Japan (Tokyo, Japan) and housed 5 per cage in a microisolator (Shin Toyo Seisakusho, Saitama, Japan) at a temperature of 23±3°C, constant humidity, and 12-h light/dark cycle. Animals had free access to tap water and standard mouse chow (Funabashi Farm, Chiba, Japan) and were given a period of 1 week for acclimatization before the experiment.
This study was carried out in accordance with the Regulations for Animal Experimentation of Shinshu University. The animal protocol was approved by the Committee for Animal Experiments at Shinshu University (Approval Number 250013) in 2013. Based on national regulations and guidelines, all experimental procedures were reviewed by the Committee for Animal Experiments and finally approved by the president of Shinshu University.
Drug AdministrationThe mice were divided into a saline control group (S), a saline+AM (SA) group, a DOX (D) group, and a DOX+AM (DA) group. Both of the DOX-treated groups (D and DA) received a single intraperitoneal injection of 15 mg/kg of DOX (Adriacin®; Kyowa Hakko Kirin, Tokyo, Japan). The AM-treated groups (SA and DA) received AM (Sigma-Aldrich, St. Louis, MO, U.S.A.) dissolved in 0.9% saline by continuous subcutaneous injection using an osmotic minipump (Alzet, Cupertino, CA, U.S.A.) at a rate of 0.05 mg/kg/min for either 3 or 7 d. The osmotic minipump was implanted under 2.5% isoflurane inhalation anesthesia. The animals in the control group received an equal volume of saline. Samples were collected 3, 7, or 14 d after DOX administration and the effects of AM were evaluated in the acute phase (day 3), the middle phase (day 7), and the chronic phase (day 14).
Alterations in DOX-Induced LethalityMice were given a single intraperitoneal injection of 20 mg/kg DOX. In the AM treated groups, AM was administered at a rate of 0.05 mg/kg/min by continuous subcutaneous injection using an osmotic minipump. The number of surviving animals was counted every day after DOX injection for 14 d. The difference in survival rate between the AM-treated and the non-AM-treated groups was examined for statistical significance using the log-rank test.
Serum Biochemical ExaminationThe mice were anesthetized with isoflurane and blood was collected from the abdominal aorta. The blood samples were transferred into 1.5-mL tubes containing serum-separating medium (Bloodsepar; IBL, Gunma, Japan). After standing at room temperature for 30 min, the samples were centrifuged (3000 rpm, 10 min) and serum samples were collected. The samples were stored at −80°C and transported on dry ice. Serum creatine phosphokinase (CK) and lactate dehydrogenase (LDH) were measured using the JSCC reference method by Oriental Yeast Co., Ltd. (Tokyo, Japan).
Morphological Changes in Cardiac TissueTissues were fixed with 4% paraformaldehyde, embedded in paraffin, and cut into sections 5 µm thick for histological examination. The paraffin sections were immersed in Hemo-De (Falma, Tokyo, Japan) for deparaffinization, and subjected to hydrophilic treatment by sequential immersion in a series of 100, 95, 80, and 70% ethanol followed by deionized water.
In hematoxylin and eosin (H&E) staining, the nucleus was colored blue with Mayer’s hematoxylin, while the cytoplasm was colored red with eosin. In Sirius-red staining, the nucleus was colored black with Weigert’s iron hematoxylin, while the cytoplasm was colored yellow and the collagen fibers were colored red31) with Van Gieson solution (picric acid and Sirius-red mixture) (Muto Kagaku, Tokyo, Japan).
The stained sections were observed under an inverted microscope (CKX41; Olympus, Tokyo, Japan).
Evaluation of Cardiac Cell DeathTerminal deoxynucleotidyl transferase deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL) staining was carried out using a Cardio TACS Kit (Trevigen, Gaithersburg, MD, U.S.A.). The deparaffinized and hydrophilicity-treated samples were washed with phosphate-buffered saline (PBS). The sections were then treated with proteinase K for antigen activation and immersed in 3% hydrogen peroxide/methanol solution to block endogenous peroxidase activity. Fragmented nuclei (indicative of cell death) were labeled with biotin and horseradish peroxidase (HRP)-conjugated streptavidin. TACS Blue Label (Trevigen) was used for staining of TUNEL-positive nuclei, and counterstaining was performed with Nuclear Fast Red (Trevigen).
The stained samples were observed under a microscope and TUNEL-positive and TUNEL-negative cells in the sections were counted.
Structural Changes in MitochondriaThe mice were anesthetized and perfusion fixation was performed from the left ventricle with a peristatic pump. The heart ventricle tissues were fixed with 2.5% glutaraldehyde and 4% osmium tetroxide, embedded in epoxy resin, and cut into ultrathin sections. The ultrathin sections were electron-stained with uranyl acetate and lead citrate, subjected to carbon shadowing, and observed with transmission electron microscopy (JEM1400; Jeol, Tokyo, Japan).
Quantification of Gene ExpressionThe heart ventricle tissues were collected and frozen in liquid nitrogen after being washed in PBS. The samples were homogenized in TRI Reagent (Molecular Research Center, Cincinnati, OH, U.S.A.) using a homogenizer (Bio-Gen PRO200; PRO Scientific, Oxford, CT, U.S.A.) to extract total RNA, after which the RNA was treated with DNase (TURBO-DNA-free Kit; Life Technologies, Carlsbad, CA, U.S.A.) to remove contaminating DNA. The RNA was then subjected to reverse transcription using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, U.S.A.). Quantitative reverse transcription polymerase chain reaction (RT-PCR) was carried out using a Step One Plus Real-Time PCR system (Applied Biosystems) with SYBR Green Realtime PCR Master Mix Plus (Toyobo, Osaka, Japan). Values were normalized relative to 18S ribosomal RNA. The primer sequences used are listed in Supplementary Table 1.
DOX-Induced Lipid PeroxidationThe cardiac ventricle tissues were collected and frozen in liquid nitrogen after being washed in PBS. The samples were stored at −80°C until use. The samples were then homogenized on ice with a Bio Masher III (Nippi, Tokyo, Japan) and malondialdehyde (MDA) was extracted. MDA was measured in cardiac tissue with a BIOXYTECH MDA Assay Kit (Percipio Biosciences, Burlingame, CA, U.S.A.).
Statistical AnalysisThe data were collected, analyzed, and reported as the mean±standard error of the mean (S.E.M.) Statistical comparisons between groups were carried out using Stat Mate statistical software (ATMS, Tokyo, Japan). The data were analyzed by the Tukey–Kramer method. In all analyses, p<0.05 was taken to indicate statistical significance.
Figure 1A shows AM and CLR gene expression in cardiac ventricle tissue on day 3 after DOX injection. The D (0.68) and DA (0.54) groups show significant decreases in CLR compared with the S group (1.00). CLR gene expression was decreased in the DA group compared with the SA group (0.93). DOX and exogenous AM administration did not alter AM gene expression on day 3 after DOX injection. Figure 1B shows AM and CLR gene expression 7 d after DOX injection. The DA (0.85) group shows significant deference of CLR compared with the S group (1.00). There was no significant difference in gene expression of AM between groups on day 7. Figure 1C shows AM and CLR gene expression 14 d after DOX injection. AM gene expression was decreased in the DA group (0.84) compared with the SA group (1.10). DOX and exogenous AM administration did not alter endogenous AM or CLR gene expression compared with the S group on day 14 after DOX injection.
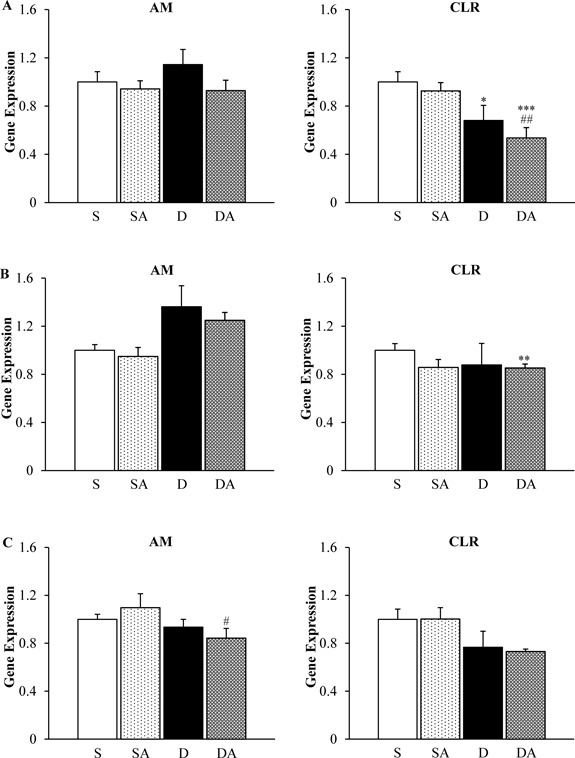
Gene expression of AM and its receptor CLR on day 3 (A), day 7 (B), and day 14 (C). The data are presented as the mean±S.E.M. * p<0.05, ** p<0.01, *** p<0.001 vs. S group. # p<0.05, ## p<0.01 vs. SA group. n=5–6/group.
Figure 2 shows the Kaplan–Meier curve for mice treated with 20 mg/kg DOX. AM treatment significantly improved survival rate (log-rank test); 9.52% of DOX-treated and 30% of DOX+AM-treated mice survived until day 14. DOX-induced lethality was observed from day 2 in the D group and from day 6 in the DA group. There were no deaths due to saline or AM injection (data not shown).

Kaplan–Meier curve of high-dose (20 mg/kg) DOX-treated mice. Circles, D group (n=21); squares, DA group (n=10). * p<0.05 vs. D group, log-rank test.
As shown in Fig. 3A, serum LDH was significantly elevated in the D (1287.7 IU/L) and DA groups (1605.3 IU/L) compared with the S (189.3 IU/L) and SA groups (210.7 IU/L) on day 3. Serum CK also shows significant elevation in the DA group (1859.0 IU/L) compared with the S (53.3 IU/L) and SA groups (63.3 IU/L) on day 3. There were no differences in either LDH or CK between the D and DA groups on day 3. Figure 3B shows significant elevation of serum CK in the D group (359.2 IU/L) compared with the S (150.6 IU/L) and SA (166.2 IU/L) groups on day 7. The DA group (255.6 IU/L) did not show significant elevation of CK. There were no differences in LDH between groups on day 7. Figure 3C shows significant elevation of serum LDH in the D group (543.5 IU/L) compared with the S (197.3 IU/L), SA (221.1 IU/L), and DA groups (361.8 IU/L) on day 14. AM treatment significantly suppressed the DOX-induced elevation of LDH on day 14. Serum CK was significantly elevated in the D group (190.0 IU/L) compared with the S (78.9 IU/L) and SA groups (97.6 IU/L) on day 14. The DA group (137.4 IU/L) did not show significant elevation of CK on day 14.
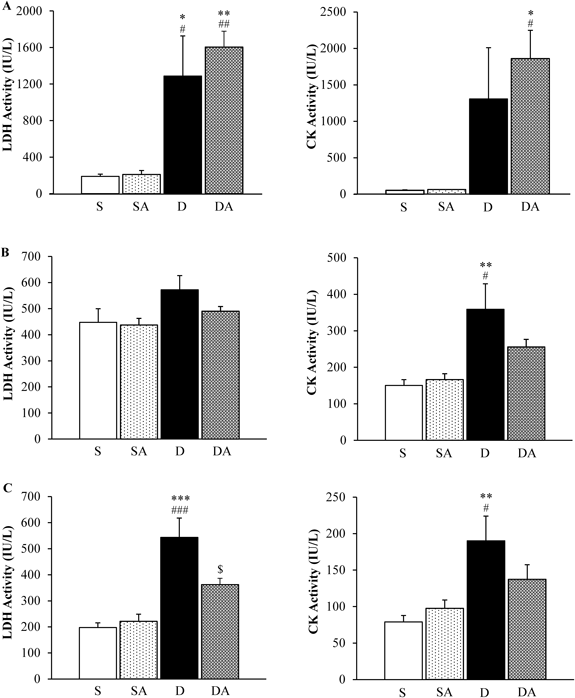
Serum LDH and CK contents on day 3 (A), day 7 (B), and day 14 (C). The data are presented as the mean±S.E.M. * p<0.05, ** p<0.01, *** p<0.001 vs. S group. # p<0.05, ## p<0.01, ### p<0.001 vs. SA group. $ p<0.05 vs. D group. n=3–4/group (day 3), n=5–6/group (day 7), n=9–10/group (day 14).
Figure 4A shows myofibrillar loss and cytoplasmic vacuolization, as shown by H&E staining, in the D group on day 7 and day 14. In the DA group, abnormality of cardiomyocytes was recovered. Figure 4B shows cardiac interstitial fibrosis, as indicated by red staining, in the D group on day 7 and day 14. Cardiac fibrosis was suppressed in the DA group. There were no differences in patterns of H&E and Sirius-red staining in ventricle tissue between any of the groups on day 3.

H&E staining (A) and Sirius-red staining (B) of the ventricle. On H&E staining, nuclei were colored blue and the cytoplasm was colored red. On Sirius-red staining, collagen was colored red, nuclei were colored black, and the cytoplasm was colored yellow. Bar: 50 µm.
As shown in Figs. 5A and B, there were no significant changes in cell death on day 3. On day 7, the rate of TUNEL-positive cells was significant elevated in the D group (46.3%) compared with the S (17.4%) and SA groups (12.0%). Administration of AM significantly reduced cardiac cell death induced by DOX (DA group: 24.9%). On day 14, significant elevation of TUNEL-positive cells was observed in the D group (3.0%) compared with the S (0.6%) and SA groups (0.8%). AM treatment significantly suppressed cardiac cell death in the DA group (0.9%).
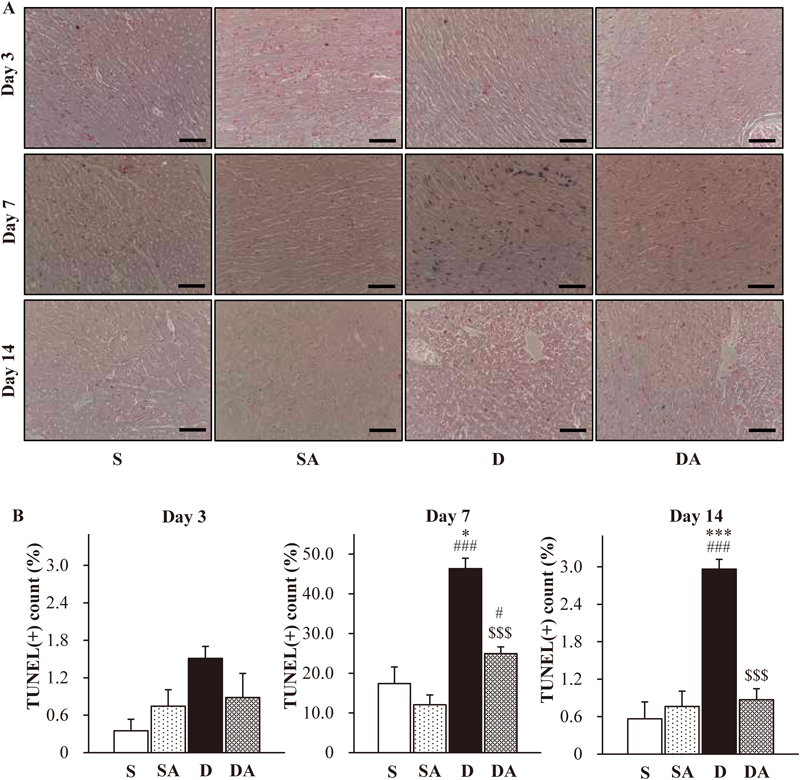
TUNEL staining of the ventricle (A). TUNEL-positive nuclei were colored dark blue and TUNEL-negative nuclei were colored red. The cytoplasm was colored pink. Bar: 50 µm. TUNEL-positive rate of the sections (B). The data are presented as the mean±S.E.M. * p<0.05, *** p<0.001 vs. S group. # p<0.05, ### p<0.001 vs. SA group. $$$ p<0.001 vs. D group. n=3–4/group (day 3), n=5–6/group (days 7, 14).
Figure 6A shows MDA content in the ventricle tissue on day 14. Figures 6B, C, and D show nicotinamide adenine dinucleotide phosphate (NADPH)-related gene expression in cardiac ventricle tissue 3 d (B), 7 d (C), and 14 d (D) after DOX injection. There were no significant differences between any of the groups.

MDA measurement on day 14 (A). Quantification of NADPH oxidase gene expression on day 3 (B), day 7 (C), and day 14 (D) in the ventricle tissue. The data are presented as the mean±S.E.M. of 4–5 independent mice.
Figure 7A shows vacuolar degeneration of mitochondria in cardiac myocyte (arrows) in the D group on 7 and 14 d after DOX injection. There was no change in mitochondrial structure on day 3. Figure 7B shows gene expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), a transcriptional coactivator of mitochondria-regulatory factors in cardiac ventricle tissue. There were no differences between any of the groups on day 3. Significant depression of gene expression of PGC-1α was observed in the D group (0.70) compared with the S (1.00) and SA (0.97) groups on day 7. AM treatment significantly recovered gene expression of PGC-1α in the DA group (0.97). On day 14, AM treatment induced a significant increase of PGC-1α (SA group; 1.66) compared with the S (1.00) and D groups (0.97).
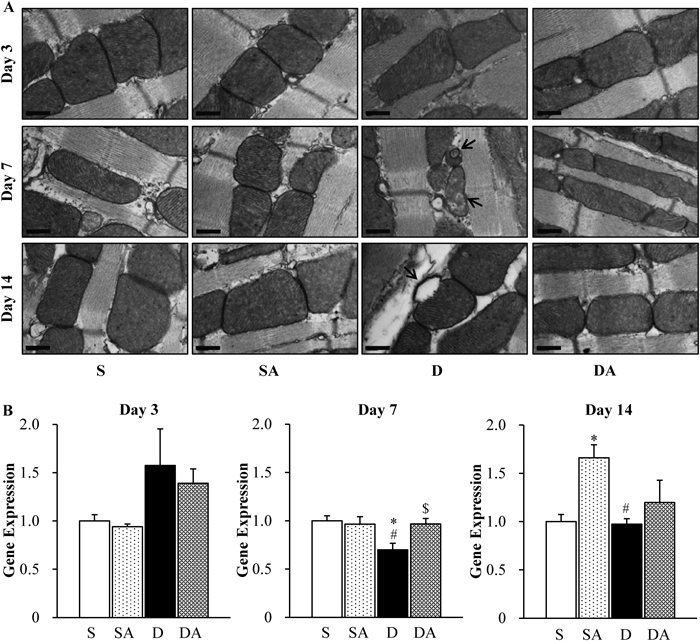
Transmission electron microscopy of cardiac mitochondria (A). Vacuolar degeneration of mitochondria (arrow). bar: 400 nm. Gene expression of PGC-1α, a transcriptional coactivator of mitochondria-regulatory factors (B). The data are presented as the mean±S.E.M of 4–5 independent mice. * p<0.05 vs. S group. # p<0.05 vs. SA group. $ p<0.05 vs. D group.
Administration of AM requires specific attention because of its short circulating half-life.32) In this study, the mice received continuous subcutaneous injection from an osmotic minipump. It has been reported previously that AM administration using an osmotic minipump is suitable for developing the functions of AM in mice.33,34)
Binding to the AM receptor (CLR) is required for expression of its bioactivity. Administration of DOX induced a significant decrease in the level of CLR gene expression on day 3, while there was no change in AM gene expression. Repression of CLR suggests hypofunction of endogenous AM. AM treatment could not compensate for reduced CLR gene expression, but exogenous AM administration may make up for AM signaling by binding to remaining CLR with increasing blood AM concentration. This kind of recovery of AM signaling in the acute phase may enhance convalescence.
Lethal heart failure is one of the most serious side effects of DOX, and can result in cessation of anticancer treatment as well as having an adverse influence on patients’ QOL. Administration of AM to mice treated with high-dose DOX (20 mg/kg) significantly improved their survival rate, which is used as an indicator of therapeutic efficacy against DOX-induced organ injury and death. These observations suggested that AM has a protective effect against serious DOX-induced side effects. Fifteen milligrams per kilogram DOX injection could not induce lethality in the mice (data not shown).
LDH is an enzyme expressed in most cell types and is used as a general index of organ damage, while CK is expressed in cardiac muscle, skeletal muscle, and brain cells and is a more specific index of muscular damage. Exogenous AM administration reduced the elevation of serum CK in DOX-treated mice on day 7 (middle phase) and day 14 (chronic phase). Elevation of serum LDH was suppressed by AM administration in the chronic phase. The results outlined above indicate that exogenous AM reduced cardiac damage induced by DOX treatment. In contrast, serum LDH and CK were increased in the D and DA groups on day 3 (acute phase). It is known that DOX strongly affects and damages the tissues that undergo active cell division, including cancer, and other healthy organs via suppression of cell proliferation through inhibition of DNA polymerase and RNA polymerase. On the other hand, DOX induces serious cardiac damage even though most adult cardiac myocytes do not divide, which suggest that the mechanism of cardiac injury is different from the mechanism of inhibition of cell division. In human patients treated with DOX, most heart failure is observed later than inhibition of cell division (several weeks to several months after treatment), which suggests that elevation of LDH and CK in acute phase is caused by non-cardiac-specific cell death.
The myocardial pathology associated with DOX treatment included myofibrillar loss, cytoplasmic vacuolization, and cardiac interstitial fibrosis in the middle and chronic phases. Furthermore, DOX induced cardiac cell death, as determined by TUNEL staining. This pathological damage was reduced by exogenous AM treatment. Thus, exogenous AM can protect the heart against DOX-induced cardiac damage.
It is generally now, it is known that risk of DOX-induced heart failure is significantly increased after a total dose of 550 mg/m2 in the patient. This is one reason that DOX-induced cardiomyopathy is observed most often in the chronic phase. Anticancer treatment is often long-term, and reduction of side effects is particularly important in the chronic phase. The mice in this study began to die 2 d after high-dose DOX injection, which suggest that acute lethal damage may occur at this stage. Serum biochemical examinations indicated increased organ damage in both the D and DA groups, which suggest that exogenous AM treatment could not alter DOX-induced non-cardiac-specific damage in the acute phase. On the other hand, suppression of LDH and CK expression in the middle and chronic phases and improvement of cardiac cell death were observed with AM treatment. These observations indicated the cardio-therapeutic effect of AM and suggested that AM administration may be a useful treatment for DOX-induced cardiomyopathy.
It is essential that we understand the mechanism of the cardiac-protection against DOX by exogenous AM. One important possibility with regard to the mechanism of cardio-protection is the antioxidative effect of AM. Lipid peroxidation is used as an indicator of oxidative stress in cells and tissues. Malondialdehyde (MDA) is the most abundant reactive carbonyl complex derived from lipid peroxides, and therefore measurement of MDA is widely used as an indicator of lipid peroxidation.35) Cardiac MDA contents were measured to evaluate DOX-induced oxidative stress, but no changes were observed in any of the groups on day 14. AM is known to regulate NADPH oxidase and to reduce oxidative stress.18,19) However, no changes were observed in gene expression of NADPH-oxidase-related factors. It is possible that the antioxidative mechanism of the action of AM has no effect on the increases in ROS production induced by DOX.
Mitochondrial regulation is on the important possibility with regard to the mechanism of cardio-protection. It is known that mitochondria control glucose and lipid metabolism, energy (ATP) production, and cytochrome c (a heme protein in the mitochondrial inner membrane)-dependent apoptosis. Because mitochondrial content in the heart is higher than in other organs, mitochondrial damage can induce serious cardiac injury. DOX was reported to induce mitochondrial malfunction, thus exacerbating cardiac conditions.7) Previous studies showed that DOX binds with the inner mitochondrial membrane and inhibits the electron transport chain,8,9) and the damage to the inner mitochondrial membrane causes release of cytochrome c and induces apoptosis.36) In electron microscopy, DOX induced vacuolar degeneration of cardiac mitochondria, which suggest mitochondrial damage (Fig. 7A, Supplementary Fig. 1). Exogenous AM treatment canceled changes in mitochondrial structure. A recent study suggested that AM regulates cardiac mitochondria through its effects on the gene expression of mitochondria-regulatory factors.22) The present study showed that the decrease in gene expression of PGC-1α induced by DOX was significantly recovered through AM treatment in the middle phase. In the chronic phase, exogenous AM administration significantly increased gene expression of PGC-1α and peroxisome proliferator-activated receptor alpha (PPARα). Gene expression of estrogen-related receptor α (ERRα) was not changed (Supplementary Fig. 2). PGC-1α is one of the most important transcriptional coactivators of mitochondrial factors related to the trichloroacetic acid (TCA) cycle, lipid β-oxidation, and the electron transport chain.37,38) PPARα and ERRα are downstream factors of PGC-1α and mainly regulate mitochondrial lipid metabolism (by PPARα), the TCA cycle, and the electron transport chain (by ERRα).39) In addition, recent studies show that AM regulates mitochondria via cAMP-cAMP response element binding protein (CREB)-PGC-1 pathway in mice,22,40) and has an anti-apoptotic effect against DOX via cAMP-dependent pathway in cultured cardiac myocytes.30) These results suggest that exogenous AM administration is able to protect the heart via regulation and protection of cardiac mitochondria in vivo.
In summary, the present study demonstrated that AM protects the heart from DOX-induced damage in vivo, which suggest that AM may be useful as a therapeutic agent for ameliorating the side effects of DOX in cancer patients. Furthermore, as AM and DOX stimulated mitochondrial changes in the heart, elucidation of these mitochondria-related mechanisms may lead to novel therapies in the future.
We are grateful to all of the staff of the Division of Instrumrntal Analysis and the Division of Laboratory Animal Reserch, Shinshu Univerity, for assisting our experiments. We wish to thank Mr. Robert Weingart for conscientious English proofreading. This study was supported by the Japan Society for the Promotion of Science (JSPS KAKENHI Grant-in-Aid for Young Scientists (B) Grant Number 26860551). This study was supported by Shinshu University (Grant-in-Aid for Exploratory Research for Young Scientists in Shinshu University).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.