2016 Volume 39 Issue 5 Pages 807-814
2016 Volume 39 Issue 5 Pages 807-814
Factor for adipocyte differentiation 24 (fad24) is a positive regulator of adipogenesis. We previously found that human fad24 is abundantly expressed in skeletal muscle. However, the function of fad24 in skeletal muscle remains largely unknown. Because skeletal muscle is a highly regenerative tissue, we focused on the function of fad24 in skeletal muscle regeneration. In this paper, we investigated the role of fad24 in the cell cycle re-entry of quiescent C2C12 myoblasts-mimicked satellite cells. The expression levels of fad24 and histone acetyltransferase binding to ORC1 (hbo1), a FAD24-interacting factor, were elevated at the early phase of the regeneration process in response to cardiotoxin-induced muscle injury. The knockdown of fad24 inhibited the proliferation of quiescent myoblasts, whereas fad24 knockdown did not affect differentiation. S phase entry following serum activation is abrogated by fad24 knockdown in quiescent cells. Furthermore, fad24 knockdown cells show a marked accumulation of p27Kip1 protein. These results suggest that fad24 may have an important role in the S phase re-entry of quiescent C2C12 cells through the regulation of p27Kip1 at the protein level.
To elucidate the molecular mechanism of adipocyte differentiation, we previously isolated 102 genes whose expression was upregulated in the early stage of adipocyte differentiation using the polymerase chain reaction (PCR)-subtraction-cloning method.1,2) Using the rapid amplification of cDNA ends (RACE) and cDNA library screening, we identified five novel genes, factor for adipocyte differentiation (fad) 24, fad49, fad104, fad123, and fad158.3–6)
The gene fad24 is the mammalian homolog of Noc3, which is involved in DNA replication in yeast. Gain-of-function and loss-of-function experiments have demonstrated that FAD24 promotes adipogenesis by controlling DNA replication.3,7) Furthermore, to identify the functions of fad24 during adipogenesis in vivo, we generated transgenic (TG) mice.8) The over-expression of fad24 increased the number of smaller adipocytes in white adipose tissue and improved glucose metabolism activity. These results strongly suggest that fad24 plays a crucial role in promoting adipocyte differentiation in vitro and in vivo. Recently, we generated fad24-deficient mice using a gene-targeting strategy and revealed that fad24 is essential for pre-implantation embryos to develop into blastocysts.9) These results indicate that fad24 is required for not only adipogenesis but also pre-implantation development.
We have previously reported that human fad24 is abundantly expressed in the skeletal muscle.3) Furthermore, fad24 promotes the proliferation of C2C12 cells which is a satellite-derived myogenic cell line.10) It was reported that a mutation of zebrafish fad24 caused muscle degeneration accompanied by leukocyte infiltration.11) These studies implicated that fad24 plays important roles in skeletal muscles. However, a histological analysis demonstrated no morphological changes including cell size and myofiber size in the femoral muscles of fad24 TG and non-TG mice.8)
Skeletal muscle is a highly regenerative tissue. Upon injury, the skeletal muscle has a remarkable ability to initiate a rapid and extensive repair process. The initial phase of muscle repair is characterized by the necrosis of damaged tissue and activation of an inflammatory response. This phase is quickly followed by the activation of satellite cells to proliferate, differentiate, and fuse leading to new myofiber formation.12–14) Several factors are involved in skeletal muscle regeneration. For example, insulin-like 6 (Insl6), an Akt-regulated myokine, is upregulated in the regenerating muscle in response to cardiotoxin (CTX)-induced injury. Furthermore, the over-expression of Insl6 stimulates satellite cell activation and accelerates muscle regeneration.15) However, it is unclear whether fad24 is involved in skeletal muscle regeneration.
In this study, we demonstrated that the expression levels of fad24 and histone acetyltransferase binding to ORC1 (hbo1), an interacting protein for FAD24, were drastically elevated at an early phase of the muscle regeneration process. In addition, the knockdown of fad24 expression impaired the S phase re-entry of quiescent C2C12 cells after serum stimulation. Furthermore, the protein level of p27Kip1 augmented with fad24 knockdown. These results suggest that fad24 has an important role in the S phase re-entry of quiescent C2C12 cells mimicking satellite cell through the regulation at the protein level of p27Kip1.
Animal experiments were performed with approval from the Committee on the Ethics of Animal Experiments in Nagoya City University.
Chemical Injury of Tibialis Anterior MuscleCTX was obtained from Sigma (St. Louis, MO, U.S.A.). A 50 µL aliquot of 20 µM CTX was injected into the tibialis anterior (TA) muscle. For histological and quantitative reverse transcription PCR (qRT-PCR) analyses, the TA muscles were harvested at various times after CTX injection.
Histological AnalysisThe TA muscles were embedded in optimal cutting temperature compound (Sakura, Tokyo, Japan) and frozen on dry ice. Cryosections (20 µm thick) were fixed in 4% paraformaldehyde, and stained with hematoxylin and eosin (H&E).
Cell Culture and Cell CountingMouse C2C12 myoblasts were cultured as previously described.10) For the differentiation experiment, medium was replaced with Dulbecco’s modified Eagle’s medium (DMEM) containing 1% fetal bovine serum (FBS). Transfection of the small hairpin RNA (shRNA) expression plasmids into C2C12 cells was performed as previously described.10) To induce C2C12 myoblasts into a state that mimicked the properties of a quiescent satellite cell, cells were cultured in serum-free DMEM for 72 h. These cells were then stimulated by incubation in DMEM containing 10% FBS.
For cell counting, C2C12 cells were trypsinized in 35 mm dishes at various time points after serum stimulation and were collected by centrifugation. Cell numbers were measured by hemocytometer.
Cell Cycle AnalysisC2C12 cells transfected with the shRNA expression plasmids were arrested in G0 phase by serum deprivation for 72 h in serum-free DMEM and then cultured in DMEM containing 10% FBS to induce re-entry into the cell cycle. These cells were collected by centrifugation, washed in phosphate-buffered saline (PBS), and fixed with cold 70% EtOH at –20°C for 4 h. The cells were resuspended in PBS supplemented with 1% bovine serum albumin (Nacalai Tesque, Kyoto, Japan) and 200 µg/mL ribonuclease (RNase) A and were incubated at 37°C for 30 min. The cells were stained with 100 µL of 10 µg/mL propidium iodide (PI; Sigma) and the DNA content of individual cells was analyzed in a flow cytometer (BD FACSVerse; Becton Dickinson, Franklin Lakes, NJ, U.S.A.) after filtering through a 100 µm nylon mesh (One Riverfront Plaza, Corning, NY, U.S.A.). Using FACSuite Software (Becton Dickinson), data from 20000 cells were collected per sample.
qRT-PCRTotal RNA extraction and reverse transcription reaction were performed as previously described.16) The qRT-PCR analyses of fad24, hbo1, myoD, and 18S ribosomal RNA (rRNA) were performed with EagleTaq Universal Master Mix with ROX (Roche Applied Science, Basel, Switzerland). The pre-designed primers and probe sets were obtained from Applied Biosystems (Foster City, CA, U.S.A.). The qRT-PCR analyses of myogenin and p27Kip1 were performed with SYBR premix Ex Taq II (TaKaRa Bio, Shiga, Japan). The specific primers for these two genes were used as previously described.17,18) An ABI PRISM 7300 sequence detection system (Applied Biosystems) was used to perform the qRT-PCR. The mixture was incubated at 50°C for 2 min and at 95°C for 10 min, and then the PCR was performed at 95°C for 15 s and at 60°C for 1 min for 40 cycles.
Western BlottingThe cells were washed with PBS and lysed in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris–HCl, 1% Nonidet-P-40, 0.1% sodium dodecyl sulfate (SDS), 0.5% deoxycholate, and 0.15 M NaCl, pH 7.5) supplemented with a protease inhibitor mixture (Nacalai Tesque). After centrifugation, the supernatant was harvested. These lysates were separated by SDS polyacrylamide gel electrophoresis. The resolved proteins were transferred to a polyvinylidene difluoride membrane and probed using primary antibody and subsequently secondary antibody conjugated with horseradish peroxidase (1 : 10000; Jackson ImmunoResearch Laboratories, West Grove, PA, or Vector Laboratories, Burlingame, CA, U.S.A.). Specific proteins were detected using an enhanced chemiluminescence system (GE Healthcare, Buckinghamshire, U.K.). Primary antibodies recognizing mouse p27Kip1 (1 : 5000, BD Transduction Laboratories) and glyceraldehydes-3-phosphate dehydrogenase (GAPDH; 1 : 2000, Acris, San Diego, CA, U.S.A.) were used.
Statistical AnalysisAnalyses were performed using Excel 2008 (Microsoft Corp.). Statistically significant differences were evaluated using two-tailed Student’s t-test. For multi-group analyses, significance was assessed using one-way ANOVA with post-hoc Tukey–Kramer honest significant difference (HSD) test. A p value of less than 0.05 or 0.01 was considered significant.
To examine the expression of fad24 during the skeletal muscle regeneration, CTX was injected into TA muscles of 9-week-old wild-type C57BL6J mice. At various time points after CTX injection, the TA muscles were collected for histological and qRT-PCR analysis. We first performed histological analyses (Fig. 1A). At day 1 after injection, the infiltration of mononuclear cells was observed. At day 3, the amount of mononuclear cells drastically increased. At day 7 after the injection, the number of mononuclear cells was reduced and that of newly formed myotubes with centrally located myonuclei was augmented. Finally, larger myotubes were formed in the regenerating regions at day 14 after CTX injection, suggesting that the skeletal muscle injury caused by CTX was repaired. In agreement with these results, the expression level of myogenic nuclear factor myoD, which has an important role in the proliferation of satellite cells in an adult skeletal muscle, increased after CTX injection as described previously15,19) (Fig. 1B).

(A) Histological analysis of the TA muscles in skeletal muscle regeneration in response to CTX injury. Cryosections of the TA muscles at indicated days after CTX injections were stained with H&E. Bars=100 µm. (B–D) The mRNA levels of myoD (B), fad24 (C), and hbo1 (D) were determined during skeletal muscle regeneration in response to CTX injury. The expression level of each gene was normalized with 18S rRNA expression. Bars indicate standard error (n=3).
Next, we examined the time course of the fad24 expression in CTX-induced muscle injury and regeneration under the above conditions. The data demonstrated that the expression level of fad24 was transiently induced 12 h after CTX injection and then rapidly decreased (Fig. 1C). We have previously reported that FAD24 interacts with HBO1 and promotes cell proliferation by controlling DNA replication.7) Therefore, we investigated the changes in hbo1 expression in skeletal muscle regeneration. Similar to fad24 expression, the level of hbo1 mRNA also quickly increased 12 h after CTX injection (Fig. 1D). These results imply that fad24 and hbo1 are involved in the early phase of skeletal muscle regeneration.
fad24 Is Not Required for Myogenic Differentiation of C2C12 CellsThe capacity for myogenic regenerative response is primarily due to a mononuclear cell population termed satellite cells.12) Adult satellite cells are muscle-specific stem cells located under the basal lamina of muscle fibers, and they are normally quiescent. In response to muscle injury, satellite cells re-enter the cell cycle, proliferate and differentiate into mature myofibers.12,20) To investigate the potential role of fad24 in muscle regeneration, we first assessed the effect of silencing fad24 in the myogenic differentiation of C2C12 myoblasts. We introduced shRNA expression plasmid for fad24 into C2C12 cells, and the expression of fad24 was determined by qRT-PCR. The expression level of fad24 was reduced by approximately 60% compared with that of control at day 6 after the induction of myogenic differentiation (Fig. 2A). Using these transfected cells, we next performed differentiation experiments. Six days after induction, control cells well differentiated and many myotubes were observed. The knockdown of fad24 did not change myotube formation compared with control cells (Fig. 2B). Furthermore, the expression levels of myogenin, a myogenic marker, were not significantly changed in the fad24-knockdown cells compared with control cells at both of 2.5 and 6 d after induction (Fig. 2C). These results suggest that fad24 is not required for myogenic differentiation of C2C12 cells.

(A) Knockdown efficiency of fad24 in C2C12 myotubes. C2C12 cells were transfected with shRNA expression plasmid for fad24 in growth medium. Scramble shRNA was used as a control. At 24-h post-transfection, the medium was replaced with differentiation medium and cultured for 6 d. The expression level of fad24 was determined and normalized with 18S rRNA expression. Bars indicated standard deviation (n=3, * p<0.01). (B) Phase-contrast images of C2C12 cells cultured with differentiation medium for 6 d. Bars=100 µm. (C) Effect of fad24 depletion on myogenin mRNA expression at 2.5 and 6 d after the induction of myogenic differentiation. The expression level of myogenin was normalized with 18S rRNA expression. Bars indicated standard deviation (n=3).
Muscle repair is characterized by discrete stages of regeneration, the activation of satellite cells within 2 h of injury, satellite cell proliferative stage at about 2–5 d following injury, differentiation, and maturation.12) It is known that the induction of myoD occurs within 2–6 h of injury, thus, corresponding to their role in the activation of the satellite cell population.12,13) Because the expression of fad24 was upregulated at 12 h after CTX injection, we next focused on the activation of satellite cells.
To elucidate whether fad24 has any roles in the activation and proliferation of quiescent satellite cells, we investigated the effect of the knockdown of fad24 on serum-induced proliferation of C2C12 cells that were arrested in G0 phase. The qRT-PCR analysis showed that the expression level of fad24 was reduced by approximately 80% in C2C12 cells transfected with shfad24 expression plasmid compared with that in control at day 3 after serum deprivation (Fig. 3A). The cells treated with control plasmid underwent an almost 16-fold increase in number by 96 h after serum stimulation (Fig. 3B). Conversely, the amount of fad24 knockdown cells were significantly decreased compared with that of control cells at 48, 72, and 96 h. This result suggested that fad24 is required for the proliferation after serum stimulation.

(A) Knockdown efficiency of fad24 in C2C12 myoblasts. C2C12 cells were transfected with shRNA expression plasmid for fad24 in growth medium. Scramble shRNA was used as a control. At 24-h post-transfection, cells were cultured in serum-free medium for 72 h. The expression level of fad24 was determined and normalized with 18S rRNA expression. Bars indicated standard deviation (n=3, * p<0.01). (B) Effect of fad24 depletion on the proliferation of C2C12 cells. The number of cells was counted at indicated times after serum-stimulation. The asterisks indicate significant differences when compared with the values for control cells (** p<0.01, * p<0.05). (C–E) Expression levels of myoD (C), fad24 (D), and hbo1 (E) after serum stimulation were determined. The expression level of each gene was normalized with 18S rRNA expression. Bars indicate standard deviation (n=3).
It has been reported that myoD expression is induced in both C2C12 myoblasts and primary satellite cells after leaving quiescence, and is required for progression into S phase when C2C12 myoblasts transitioned out of quiescence.21) Therefore, we examined the alteration in myoD expression in C2C12 cells after serum stimulation. The expression level of myoD was upregulated at 3 h (Fig. 3C). Next, we examined variations in fad24 expression in C2C12 cells post serum stimulation. Similar to that of myoD, the expression level of fad24 mRNA increased at 3 h following stimulation (Fig. 3D). As shown in Fig. 3E, the expression level of hbo1 mRNA was also elevated at 3 h. These results suggested that fad24 and hbo1 might be involved in the activation of C2C12 myoblasts as well as myoD.
S Phase Entry Is Inhibited by fad24 Knockdown in Quiescent C2C12 CellsTo investigate the effect of fad24 knockdown on cell cycle progression, PI-staining of C2C12 cells was performed. The expression of fad24 was reduced by approximately 70% in C2C12 cells transfected with a shfad24 expression plasmid compared with control values at day 3 after serum deprivation (Fig. 4A). Flow cytometric analysis of the DNA content revealed that the knockdown of fad24 did not have significant influence on the histograms depicting cell cycle profiles before serum deprivation (Fig. 4B; see GM). After serum deprivation for 72 h, more than 90% of shfad24-transfected cells and control cells were arrested in the G0/G1 phase and less than 5% of the cells were in the S phase (Figs. 4B, C; see 0 h). Cells were then released from G0 phase by the addition of growth medium containing 10% FBS. Sixteen hours after serum stimulation, approximately 50% of the cells exited quiescence in control cells, whereas the knockdown of fad24 reduced the amount of cells that left quiescence by 25% (Figs. 4B, C; see 16 h). Compared with control cells, fad24-knockdown exhibited a significant reduction in S phase cells at 16 h after serum stimulation (Fig. 4D). Thus, we concluded that fad24 was required for re-entry into S phase and cell proliferation following serum stimulation.
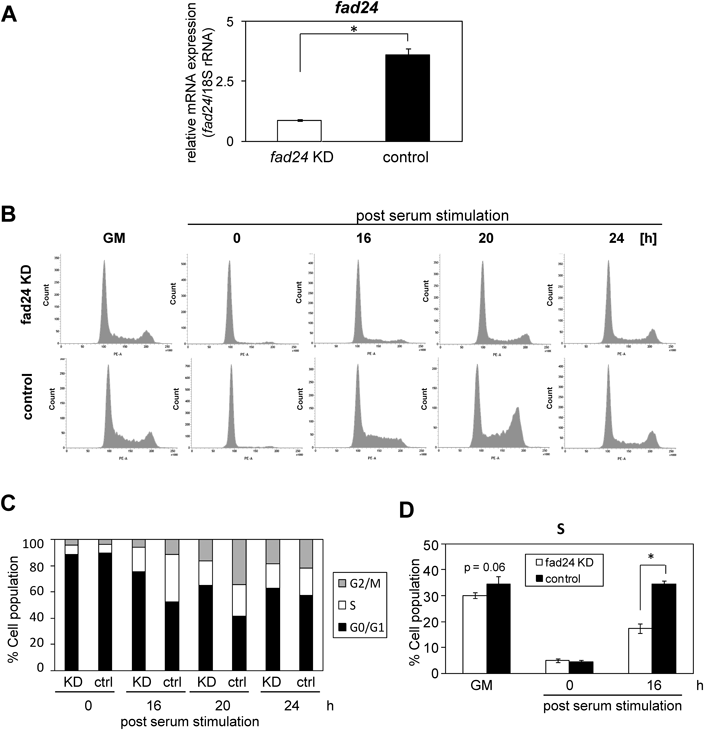
(A) Knockdown efficiency of fad24 in C2C12 myoblasts. C2C12 cells were transfected with shRNA expression plasmid for fad24 in growth medium. Scramble shRNA was used as a control. At 24-h post-transfection, cells were cultured in serum-free medium for 72 h. The expression level of fad24 was determined and normalized with 18S rRNA expression. Bars indicated standard deviation (n=3). (B–D) Effect of fad24 depletion on S phase entry in C2C12 myoblasts. C2C12 cells transfected with shRNA expression plasmids were arrested in G0 phase by serum deprivation for 72 h and were then stimulated by incubation in DMEM containing 10% FBS. These cells were harvested at each point, and analyzed by flow cytometry after PI staining. GM represents the cells cultured in growth medium before serum deprivation. Representative histograms depicting cell cycle profiles (B), quantifications of the histograms (C), and the population of cells in the S phase (D) are shown. Bars indicate standard deviation (n=3, * p<0.01).
It has been reported that myoD helps satellite cells to enter into the first round of DNA replication after transitioning out of quiescence.21) Therefore, we next investigated whether fad24 has a role in the regulation of the expression of myoD after serum stimulation. As shown in Fig. 5A, the knockdown of fad24 did not affect the myoD expression compared with control cells.

(A) Effect of fad24 depletion on myoD mRNA expression. C2C12 cells were transfected with shRNA expression plasmid for fad24 in growth medium. Scramble shRNA was used as a control. Cells were cultured in serum-free medium for 72 h at 24-h post-transfection, and were then stimulated by incubation in DMEM containing 10% FBS. The mRNA expression level of myoD at each point after serum stimulation was determined and normalized with 18S rRNA expression. Bars indicated standard deviation (n=3). (B) Effect of fad24 depletion on p27Kip1 mRNA expression. C2C12 cells were transfected with shRNA expression plasmid for fad24 in growth medium. Scramble shRNA was used as a control. At 24-h post-transfection, cells were cultured in serum-free medium for 72 h. The mRNA expression level of p27Kip1 was determined and normalized with 18S rRNA expression. Bars indicated standard deviation (n=3). (C) Effect of fad24 depletion on the protein level of p27Kip1. C2C12 cells transfected the shRNA expression plasmids were arrested in G0 phase by serum deprivation for 72 h and were then stimulated by incubation in DMEM containing 10% FBS. These cells were harvested, and analyzed by Western blotting (upper panel). Intensities of signal from p27Kip1 were quantified by NIH-Image software, and normalized to GAPDH (lower panel). Bars indicate standard deviation (n=3). p-Value was determined by one-way ANOVA with post-hoc Tukey–Kramer HSD test (# p<0.01 vs. control cells, $ p<0.05 vs. control cells at 0 h). Western blot shows the representative results in at least two independent experiments.
Transitions from one phase of the cell cycle to the next are driven by the activities of cyclin/cyclin-dependent kinase (CDK) complexes and cyclin/CDK inhibitors (CKIs), which modulate the activities of the complexes. p27Kip1, one of the CKIs, regulates the transition from G0, through G1, into S phase.22) p27Kip1 protein levels are elevated in quiescent cells and decrease following mitogen stimulation and entry into G1.23) We next investigated the effect of the knockdown of fad24 on the expression level of p27Kip1. C2C12 cells transfected with shRNA expression plasmids were arrested in G0 phase by serum deprivation for 72 h and were then stimulated by incubation in DMEM containing 10% FBS. As shown in Fig. 5B, the knockdown of fad24 did not significantly change the p27Kip1 mRNA expression level in quiescent C2C12 cells although the amount is slightly higher in knockdown cells. On the other hand, fad24 knockdown showed a marked accumulation of p27Kip1 protein in quiescent C2C12 cells (Fig. 5C; see 0 h). In control cells, the protein level of p27Kip1 was significantly decreased at 8 h after serum stimulation, suggesting that cells re-entered the cell cycle. The protein level of p27Kip1 in fad24 knockdown cells was still higher than that of control cells at 8 h post serum stimulation (Fig. 5C; see 8 h). These results indicate that the knockdown of fad24 elevates the protein expression level of p27Kip1.
Previously, we reported that human fad24 was abundantly expressed in skeletal muscles.3) In addition, we demonstrated that fad24 positively regulated the cell proliferation of C2C12 myoblasts.10) However, the function of fad24 in the skeletal muscle remained largely unknown.
In the present study, we observed that the expressions of fad24 and hbo1 were upregulated at 12 h after CTX injection (Fig. 1C). Therefore, we hypothesized that fad24 is involved in muscle regeneration. The knockdown of fad24 did not change myotube formation compared with control cells (Fig. 2). Conversely, the knockdown of fad24 exhibited a significant reduction in S phase cells at 16 h after serum stimulation (Fig. 4). These results indicate that fad24 is essential for the transition to S phase of quiescent myoblasts arrested in G0 phase. More elaborate analyses using satellite cells derived from the skeletal muscle-specific fad24 knockout mice are required to clarify the physiological role of fad24 in muscle regeneration.
The early stages of muscle regeneration are characterized by muscle necrosis, accumulation of inflammatory cells, and the activation of satellite cells. In addition to the satellite cells, infiltrating immune cells have important roles in the regulation of muscle regeneration.13,24) M1 macrophages and neutrophils accumulate at the site of skeletal muscle injury and secrete T helper 1 (Th1) cytokines, which affect the proliferation and migration of muscle cells. It was reported that macrophages infiltrated into injured region on day 2 and reached a peak on day 3 after CTX-injection.24) On the other hand, the expression level of fad24 was elevated much earlier (Fig. 1C), indicating that fad24 functioned without existence of macrophages. However, neutrophils were known to be infiltrated earlier than macrophage infiltration.24) Therefore, it is possible that fad24 functions in the inflammatory cells, especially in the neutrophils. Further analyses, including immunohistochemical studies, and also the analyses using primary immune cells isolated from injured skeletal muscles, are required to examine the relationship of the function of fad24 and inflammatory response after muscle injury. Since the roles of fad24 in dying cells during necrosis are unclear, this issue also should be solved in the next step.
Following the induction of adipocyte differentiation, growth-arrested 3T3-L1 preadipocytes re-enter the cell cycle and undergo several rounds of cell division during the first 3 or 4 d—a phenomenon referred to as mitotic clonal expansion (MCE).25) We previously reported that FAD24 acted in concert with HBO1 to promote MCE by controlling DNA replication.7) In this study, we showed that fad24 is a positive regulator of S phase re-entry of quiescent C2C12 myoblasts. Considering these results, fad24 might be crucial for cell cycle re-entry of many types of cells. Furthermore, it is possible that HBO1 acts in concert with FAD24 in the activation of satellite cells, although the role of hbo1 in muscle regeneration is unclear.
The activation of satellite cells is regulated by several factors.14) We focused on p27Kip1, which inhibits the transition from G0 phase to S phase.26) We observed that the expression of p27Kip1 protein was enhanced by fad24 knockdown (Fig. 5C). The abundance of p27Kip1 is believed to be controlled by multiple mechanisms that operate at the level of the synthesis, degradation, and localization of this protein. The ubiquitin–proteasome pathway contributes to control the protein level of p27Kip1 by mediating the degradation of the protein. The ubiquitylation of p27Kip1 at the G0–G1 transition occurs in the cytoplasm.27) Therefore, the translocation of p27Kip1 from the nucleus to the cytoplasm in a chromosomal region maintenance 1-dependent manner is necessary for its degradation at the G0–G1 transition.28,29) We have previously revealed that FAD24 localized to the nucleolus and nuclear speckles.3) In addition, FAD24 interacts with HBO1 and affects the recruitment of HBO1 to origins of DNA replication.7) Therefore, it is possible that FAD24 forms a complex with p27Kip1 in the nucleus and regulates the translocation of p27Kip1. In addition, since it is reported that the phosphorylation of p27Kip1 affects its localization and stability,30) fad24 might regulate the phosphorylation of p27Kip1 in the nucleus. Future studies are needed to evaluate the molecular mechanism of fad24 regulating the protein level of p27Kip1.
In conclusion, our present findings provide evidence that fad24 is required for cell cycle progression in S phase and is involved in the expression of p27Kip1 protein in quiescent C2C12 cells. Further analysis of fad24 should help us understand the molecular mechanism employed at the early phase of muscle regeneration.
This work was supported in part by Grants from the Japan Society for the Promotion of Science (JSPS).
The authors declare no conflict of interest.