2016 Volume 39 Issue 5 Pages 823-831
2016 Volume 39 Issue 5 Pages 823-831
Asiasarum root (roots and rhizome of Asiasarum sieboldii or A. heterotropoides var. mandshuricum) has been frequently used in traditional Chinese medicinal formulas for the management of oral malodor syndrome caused by periodontal disease. However, there are no scientific reports concerning these effects and the mechanism of action. The objective of this study was to examine the inhibitory effects of Asiasarum root and its constituents on oral malodor syndrome and periodontal disease. A 50% ethanolic extract of Asiasarum root (AR-ext) showed L-methionine γ-lyase (METase) inhibitory activity at a concentration of 200 µg/mL, and inhibited interleukin (IL)-1β-stimulated matrix metalloproteinase (MMP)-1 secretion from human gingival fibroblasts (HGFs) at a concentration of 10 and 50 µg/mL without cytotoxic effects. Activity-guided fractionation of the AR-ext suggested that METase inhibitory activity was attributable to a mixture of linoleic and oleic acid, because these unsaturated fatty acids showed weak METase inhibitory activities. Similar fractionation using MMP-1 secretion inhibitory activity led to the isolation of two unsaturated fatty acid amides, (2E,4E,8Z,10E)-N-(2-methylpropyl)dodeca-2,4,8,10-tetraenamide (1) and (2E,4E,8Z,10Z)-N-(2-methylpropyl)dodeca-2,4,8,10-tetraenamide (2), as active constituents with inhibitory activity on MMP-1 secretion from HGFs. To elucidate the inhibition mechanism on MMP-1 secretion, the effect of 2 on mitogen-activated protein kinase (MAPK) phosphorylation was examined. Western blotting analysis revealed that 2 (10 µM) reduced the phosphorylation of p38 and c-Jun-N-terminal kinase. These results suggested that 2 suppresses intracellular MMP-1 expression and MMP-1 secretion from IL-1β-stimulated HGFs by down-regulation of MAPK phosphorylation.
Many people suffer from oral malodor syndrome, a major cause of which is recognized to be periodontal disease—a chronic inflammatory disorder triggered by subgingival bacterial infection.1) As part of our ongoing studies on novel agents from natural sources based on literature searches on traditional Chinese medicines, the target of this study was to find a novel oral care agent for the prevention and treatment of periodontal disease and oral malodor syndrome. Since ancient times in China, there have been many descriptions on the cause and treatment of oral malodor syndrome. Our previous studies on the history of pharmacy2) revealed that Asiasarum root was frequently used for the treatment of oral malodor syndrome caused by periodontal disease. Based on further literature searches on traditional Chinese medicines, it was found that Asiasarum root was used for the treatment of oral malodor syndrome in ancient Chinese herbal books. Asiasarum root is considered to be the roots or rhizome of Asiasarum sieboldii F. MAEKAWA or A. heterotropoides F. MAEKAWA var. mandshuricum F. MAEKAWA (Aristolochiaceae). Although anti-inflammatory activity,3) analgesic activity,4) and antibacterial activity5) against Streptococcus mutans, a microorganism that causes caries,6) are known biological activities of Asiasarum root, there have been no reports related to oral malodor syndrome. Periodontal disease is recognized as a major cause of oral malodor syndrome. Therefore, in this study, we examined the effect of Asiasarum root on periodontal disease.
Porphyromonas gingivalis is the best-known subgingival bacteria, and P. gingivalis infection affects tooth-supporting tissue and results in the progressive destruction of gingival connective tissue.7) Type 1 collagen is the main extracellular matrix component of periodontal tissue, thus collagen degradation is regarded as one of the key factors in destruction of the periodontium. In periodontal disease, matrix metalloproteinases (MMPs) play an important role in collagen degradation. Gingival fibroblasts naturally produce MMPs, the synthesis of which is stimulated by periodontal bacteria such as P. gingivalis and their products.8)
MMPs are a family of structurally related matrix degrading enzymes that are associated with various destructive processes, including inflammation, tumor invasion, and periodontitis,8–10) and are subdivided in different types comprising at least 20 members.9–11) Among these, MMP-1, which is secreted from human gingival fibroblasts (HGFs), is mainly responsible for the degradation of periodontal collagen in the progression of periodontal disease.12) In HGFs, the inflammatory mediator interleukin (IL)-1β induces the production of MMP-1 by activating intracellular signal transcription pathways including p38 and c-Jun-N-terminal kinase (JNK) mitogen-activated protein kinase (MAPK).13) IL-1β also induces MMP-1 production in cultured HGFs, increasing the amount of MMP-1 released into the culture medium.13,14) The amount of MMP-1 released into the culture medium was 20–50 times the amount of MMP-1 expressed in HGFs14); therefore, we used IL-1β-stimulated HGFs in this study. It is considered that agents that inhibit the secretion of MMP-1 into the culture medium from HGFs may be useful for the prevention and treatment of periodontal disease and oral malodor syndrome via the inhibition of destruction of gingival connective tissue. Although it has been reported that myricetin,15) theaflavins,16) and Lupinus albus seed extract17) showed inhibitory activities on MMP-1 expression or MMP-1 secretion from HGFs, further searches for novel agents with MMP-1 secretion inhibitory activity are in progress.
Oral malodor is caused by volatile sulfuric compounds, such as methylmercaptan, which have unpleasant odors. Methionine is converted to methylmercaptan by L-methionine γ-lyase (METase), which is present in anaerobic bacteria such as P. gingivalis in the oral cavity.18,19) It has been reported that methylmercaptan relates to the progress of periodontal disease.19,20) Therefore, it was suggested that agents with METase inhibitory activity may be useful for the prevention and treatment of oral malodor syndrome and periodontal disease. METase from Pseudomonas putida is commercially available and has been widely used in other reports.21–23) Thus, this enzyme was also used for our study. Zinc chloride is a known METase inhibitor and used in toothpaste and mouthwash.24) Myrsinoic acid B with METase inhibitory activity was isolated from Myrsine seguinii25); however, few other METase inhibitors from natural sources are known.
In this paper, we examined the effects of a 50% ethanolic extract of Asiasarum root (AR-ext) on P. gingivalis, METase activity, and MMP-1 secretion from IL-1β-stimulated HGFs. Moreover, activity-guided fractionation of AR-ext led to the identification of active compounds.
L-Methionine and METase were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). 3-Methyl-2-benzothiazolinone hydrazone and pyridoxal 5′-phosphate were purchased from Sigma-Aldrich Co. (St. Louis, MO, U.S.A.). Recombinant human IL-1β was purchased from Pepro Tech, Inc. (Rocky Hill, NJ, U.S.A.). An antibody against MMP-1 and horseradish peroxidase-conjugated anti-goat immunoglobulin G (IgG) secondary antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Antibodies against p38 MAPK, JNK, phosphor-p38 MAPK, phosphor-JNK, and β-actin were purchased from Cell Signaling Technology. An enhanced chemiluminescence (ECL) Plus Western blotting detection kit and ECL anti-rabbit IgG, horseradish peroxidase-linked species-specific whole antibody were obtained from GE Healthcare UK Ltd. (Little Chalfont, U.K.). Other chemical and biochemical reagents were of reagent grade and were purchased from Wako Pure Chemical Industries, Ltd. or Nacalai Tesque, Inc. (Kyoto, Japan) unless otherwise noted.
Plant Materials and ExtractionAsiasarum root (roots or rhizome of A. heterotropoides var. mandshuricum, production number: 004313001) produced in China was purchased from Tochimoto Tenkaido Co., Ltd. (Osaka, Japan) in March 2012. The voucher specimen of Asiasarum root is deposited in Kindai University.
Asiasarum root (1 kg) was extracted with 50% ethanol (EtOH) (10 L) for 2 h under reflux. The extract was evaporated under reduced pressure and then lyophilized to give a 50% EtOH extract of Asiasarum root (AR-ext) with a 12.6% yield.
Fractionation of AR-extEach fraction was used for in vitro assays of inhibition of METase activity and MMP-1 secretion activity. A suspension of AR-ext (130 g) in water (1.3 L) was extracted with ethyl acetate (EtOAc, 1.3 L×5). Evaporation of solvent from the EtOAc extract under reduced pressure gave an EtOAc-soluble fraction (12.3 g; yield from AR-ext: 9.5%, METase inhibition %; 17% at 50 µg/mL, 36% at 200 µg/mL). The aqueous layer was evaporated under reduced pressure and then lyophilized to give a water-soluble fraction (116.7 g; 89.8%, METase inhibition %; 7% at 50 µg/mL, 9% at 200 µg/mL). A portion of the EtOAc-soluble fraction (10 g) was submitted for column chromatography over 500 g of silica gel (Merck No. 1.09385 silica gel 60, 3.3×30 cm). Elution with hexane and EtOAc in increasing proportions, and then with methanol (MeOH) monitored with TLC [Merck No. 1.05715 silica gel 60 F254, hexane–EtOAc (2 : 1, v/v), detection; UV and 10% H2SO4 followed by heating] gave 30 chromatographic fractions of 500 mL each. TLC analysis of the collected fractions allowed us to assemble them into five fractions (Fr. A to E). Fraction A [elution solvent: hexane–EtOAc (50 : 1) to (10 : 1), yield: 2.6 g, METase inhibition %; 4% at 12.5 µg/mL, 4% at 50 µg/mL], Fr. B [hexane–EtOAc (5 : 1), 1.6 g, METase inhibition %; 2% at 12.5 µg/mL, 13% at 50 µg/mL], Fr. C [hexane–EtOAc (5 : 1) to (2 : 1), 2.1 g, METase inhibition %; 15% at 12.5 µg/mL, 36% at 50 µg/mL], Fr. D [hexane–EtOAc (1 : 1), 0.7 g, METase inhibition %; 17% at 12.5 µg/mL, 41% at 50 µg/mL], and Fr. E (MeOH, 2.4 g, METase inhibition %; 2% at 12.5 µg/mL, 14% at 50 µg/mL), Fr. C and Fr. D showed three spots (Rf-value: 0.21, 0.34, and 0.50) on TLC [hexane–EtOAc (2 : 1, v/v)]. These spots in Fr. D were more ambiguous than in Fr. C. Repeated preparative TLC [hexane–EtOAc (2 : 1, v/v)] of Fr. C (300 mg) gave Fr. C-1 (single spot at Rf 0.50, 79 mg, METase inhibition %; 20% at 12.5 µg/mL, 45% at 50 µg/mL), Fr. C-2 (single spot at Rf 0.34, 102 mg, METase inhibition %; 5% at 12.5 µg/mL, 5% at 50 µg/mL), and Fr. C-3 (single spot at Rf 0.21, 10 mg, METase inhibition %; 3% at 12.5 µg/mL, 8% at 50 µg/mL).
Using GC-MS analysis using a Intertcap Pure Wax capillary column (0.25 mm i.d.×60 m) on a Agilent 7890GC/5975MSD after methylation, the active Fr. C-1 was found to be comprised of 35% linoleic acid, 25% palmitic acid, and 20% oleic acid. Preparative HPLC [CHIRALPAK® IA, 10 i.d.×250 mm, Daicel Corporation, mobile phase: hexane–EtOAc (9 : 1, v/v), 2.0 mL/min, detection UV 254 nm] of Fr. C-2 gave compounds 1 (14 mg, retention time; 8 min, colorless powder, yield 0.24% from AR-ext) and 2 (14 mg, retention time; 9 min, colorless powder, yield 0.24% from AR-ext). On the basis of NMR spectral data (1H-NMR, 13C-NMR, and several two dimensional (2D)-NMR) analysis and comparison of these physicochemical data with reported data,26) 1 and 2 were identified as known compounds (2E,4E,8Z,10E)-N-(2-methylpropyl)dodeca-2,4,8,10-tetraenamide and 2E,4E,8Z,10Z)-N-(2-methylpropyl)dodeca-2,4,8,10-tetraenamide, respectively.
Antibacterial Activity against P. gingivalisAntibacterial activity of AR-ext against P. gingivalis was assessed using the Kirby–Bauer disk diffusion test in accordance with the method of Oh et al.27) Test samples were dissolved in dimethyl sulfoxide (DMSO) and then diluted with sterile distilled water to an appropriate concentration. P. gingivalis (Coykendall et al.) Shah and Collins (ATC C® 33277™) strain was obtained from the American Type Culture Collection (Manassas, VA, U.S.A.).
METase Inhibitory ActivityMETase activity was measured in accordance with the methods of Esaki and Soda21) and Takakura et al.22) with minor modifications. Test samples were dissolved in DMSO and then diluted with 50 mM potassium phosphate buffer (PB; 50 mM KH2PO4, 50 mM K2HPO4, pH 8.0) to an appropriate concentration. The final concentration of DMSO was 5% (v/v). In the control groups, 5% (v/v) DMSO/PB was used instead of the sample solution. METase was dissolved in 50 mM PB (pH 7.2) containing 1 mM ethylenediaminetetraacetic acid (EDTA), 20 mM pyridoxal-5′-phosphate, and 0.01% β-mercaptoethanol and stored at −80°C (10 U/mL METase). METase (10 U/mL) was diluted with PB (pH 7.2) containing 1 mM EDTA, 20 mM pyridoxal-5′-phosphate, and 0.01% β-mercaptoethanol to 1.0 U/mL.
A mixture of 80 µL of test sample solution, 160 µL of 6.25 mM L-methionine (final concentration: 2.5 mM), and 160 µL of 25 µM pyridoxal 5′-phosphate (final concentration: 10 µM) was incubated at 37°C for 10 min. Ten microliters of 1.0 U/mL METase solution (final concentration: 25 mU/mL) was added, and the mixture was incubated at 37°C for 10 min. The reaction was stopped by the addition of 50 µL of 50% (w/v) trichloroacetic acid. The mixture was diluted with 920 µL of sodium acetate buffer (1 M CH3COOH, 1 M CH3COONa, pH 5.2) and then 370 µL of 0.1% (w/v) 3-methyl-2-benzothiazolinone hydrazone hydrochloride/1 M sodium acetate buffer (pH 5.2) (final concentration: 0.02%) was added to the mixture. The mixture was incubated at 50°C for 30 min, allowed to stand for 30 min at room temperature, and then the optical density (OD) was measured at 320 nm using a spectrophotometer (UV-2450, Shimadzu, Kyoto, Japan) and the amount of α-ketobutyrate produced was determined.
Cell CultureHGFs obtained from human gingiva was purchased from ScienCell Research Laboratories, Ltd. (Carlsbad, CA, U.S.A.) in December 2011. HGFs were precultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (Nichirei Biosciences Inc., Tokyo, Japan) at 37°C in a humidified CO2-controlled (5%) incubator. HGFs from passages 3 to 8 were used for the experiments.
Assay of Cell ViabilityThe cell viability was determined using a 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-8) assay with a commercial kit (Tetra Color ONE, Seikagaku Corporation, Tokyo, Japan). Test samples were dissolved in DMSO and then diluted with DMEM to an appropriate concentration. In the control group, 0.2% (v/v) DMSO/DMEM solution was used instead of sample solution. The final concentration of DMSO was 0.2% (v/v). Briefly, 1×104 cells in medium (100 µL) were placed in a 96-well microplate. After a 24-h incubation, several concentrations of samples in the medium (100 µL) were added. After incubation for 48 h, the medium was replaced with new medium (10% WST-8/DMEM, 100 µL). After incubation for 1 h, the resulting amount of formazan was determined photometrically at 450 nm using a microplate reader. Cell viability was expressed as a percentage of the control cells.
IL-1β StimulationIL-1β was added to the medium in the vehicle control and test groups. IL-1β was dissolved in 10 mM Tris–HCl (pH 8.2) containing 0.1% bovine serum albumin (BSA) to 0.1 mg/mL and then diluted with DMEM to 1 µM final concentration. HGFs (1.0×105 cells/2 mL) were seeded in 6-well plates and then cultured until confluent. The cells were washed twice with phosphate-buffered saline (PBS, pH 7.4) and treated with test samples and IL-1β at 48 h after seeding. Test samples were dissolved in DMSO and then diluted with serum-free medium to an appropriate concentration. The final concentration of DMSO was 0.2% (v/v). In the control and vehicle control groups, DMSO solution was used instead of the sample solution.
Western Blotting Analysis for MMP-1 in Culture MediumAliquots of the HGF culture medium were centrifuged at 12000×g for 15 min at 4°C. The supernatant was boiled for 4 min in sodium dodecylsulfate (SDS)-containing sample buffer (Bio-Rad Laboratories, Hercules, CA, U.S.A.) containing 5% (v/v) β-mercaptoethanol and then chilled on ice. An equal volume of resulting supernatant was separated by 10% SDS-polyacrylamide gel electrophoresis (PAGE). The separated proteins were transferred onto polyvinylidene fluoride (PVDF) membranes (Bio-Rad Laboratories). The membranes were blocked overnight at 4°C with 5% (w/v) skim milk in Tris-buffered saline (TBS; 25 mM Tris, 137 mM NaCl, 2.68 mM KCl) containing 0.1% Tween 20 and then incubated with anti-MMP-1 antibody at 1 : 400 dilution for 1 h at room temperature. Subsequently, the membranes were incubated with horseradish peroxidase-conjugated anti-goat IgG secondary antibody at 1 : 4000 dilution for 1 h at room temperature. The resulting proteins were detected using a ECL-Plus Western blotting detection reagents kit, and visualized with a ChemiDoc XRS Plus System (Bio-Rad Laboratories Inc.). Relative band density was determined by Quantity One 1-D Analysis Software (Version. 4.6.7, 2008; Bio-Rad Laboratories Inc.).
Western Blotting Analysis for MAPKsCultured HGFs were washed twice with pre-cooled PBS (pH 7.4) and then lysed with a cell lysis buffer (Cell Signaling Technology, Danvers, MA, U.S.A.) containing 1 mM phenymethanesulfonyl fluoride. The lysates were centrifuged at 12000×g for 15 min at 4°C. The supernatant was subjected to Western blotting analysis, as described above. Protein concentrations of samples were determined using DC protein assay reagent (Bio-Rad Laboratories Inc.) with bovine serum albumin (Bio-Rad Laboratories Inc.) as a standard. Equal amounts of samples were separated by 10% SDS-PAGE and transferred onto PVDF membranes. The membranes were subsequently blocked with 5% (w/v) skim milk in TBS containing 0.1% Tween 20 or Blocking One-P overnight at 4°C and then incubated with the indicated antibodies. Antibodies against p38, phospho-p38, JNK, phospho-JNK, and β-actin were used at 1 : 1000 dilution. The membranes were exposed to horseradish peroxidase (HRP)-conjugated anti-rabbit IgG secondary antibody at 1 : 4000 dilution. The resulting proteins were detected using ECL Plus and analyzed with a ChemiDoc XRS Plus System, as described above.
Synthesis of Amides (3, 4, and 5)According to the literature,28) phosphonoacetic acid amide X was prepared by the condensation of diethylphosphonoacetic acid and isopropylamine. To a solution of X (0.2 mM) in tetrahydrofuran (THF) was added aldehyde (0.22 mM; as shown in Fig. 9), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (0.24 mM), and lithium chloride (LiCl) (0.22 mM), successively. The mixture was stirred for 15 h at room temperature. The reaction mixture was concentrated under reduced pressure, and then purified by silica gel column chromatography to give conjugated unsaturated fatty acid amides (3 and 4). Compound 5 was prepared according to the literature29) as shown in Fig. 9.
Statistical AnalysisEach experiment was performed in triplicate unless otherwise noted. Intergroup differences were evaluated by one-way ANOVA followed by Dunnett’s multiple comparison test. Statcel3 software (OMS Publishing Inc., Saitama, Japan, 2011) was used for the statistical analyses.
AR-ext showed no antibacterial activity against P. gingivalis at a concentration of 2 mg/mL (maximum amount of AR-ext on a paper disk was 40 µg).
METase Inhibitory Activity and IL-1β-Stimulated MMP-1 Secretion Inhibitory Activity of AR-extAR-ext showed significant METase inhibitory activity at concentrations of 200 and 500 µg/mL, as shown in Table 1. AR-ext dose-dependently inhibited IL-1β-stimulated MMP-1 secretion from HGFs at concentrations of 10 and 50 µg/mL, as shown in Fig. 1. In order to identify the active constituents of AR-ext, activity-guided fractionation was carried out using assays of METase inhibitory activity and IL-1β-stimulated MMP-1 secretion inhibitory activity. Fractionation of AR-ext by solvent extraction gave EtOAc- and water-soluble fractions.
| Samples | Concentration (µg/mL) | OD (×1000)a) at 320 nm | Inhibition (%) |
|---|---|---|---|
| Control | 364±4 | — | |
| AR-ext | 200 | 314±6** | 14 |
| 500 | 250±7** | 31 |
a) OD: optical density. Each value represents the mean±S.E. of 5 experiments. Significantly different from control group, ** p<0.01.

Fibroblasts were exposed to IL-1β and then cultured in serum-free medium containing test samples for 48 h. MMP-1 protein levels in the culture medium were assessed by Western blotting analysis using an antibody against human MMP-1. The blot is representative of three separate experiments and represents a single immunoblot.
For METase inhibitory activity, the individual methods of activity-guided fractionation are described in Materials and Methods. The EtOAc-soluble fraction showed more potent activity than the water-soluble fraction. Repeated chromatography of the EtOAc-soluble fraction, which showed significant activity in the assay, afforded an active oil, which showed a single spot on TLC. NMR spectral data suggested that the oil was a mixture of several fatty acids. Using gas chromatography after methylation, the active oil was found to be comprised 35% linoleic acid, 25% palmitic acid, and 20% oleic acid. Authentic compounds of these three detected fatty acids were assayed to find which component was responsible for the inhibition. As shown in Table 2, linoleic acid and oleic acid, major unsaturated acid components of the oil, dose dependently showed a weak activity in comparison with saturated palmitic acid.
| Samples | Concentration (µM) | OD (×1000)a) at 320 nm | Inhibition (%) |
|---|---|---|---|
| Control | — | 370±3 | — |
| Linoleic acid | 15 | 312±4** | 15 |
| 20 | 256±18** | 31 | |
| 25 | 234±11** | 37 | |
| 30 | 205±5** | 44 | |
| Oleic acid | 15 | 313±24** | 15 |
| 20 | 293±2** | 21 | |
| 25 | 283±9** | 24 | |
| 30 | 265±11** | 28 | |
| Palmitic acid | 15 | 309±5** | 16 |
| 20 | 320±9** | 13 | |
| 25 | 297±7** | 20 | |
| 30 | 314±4** | 15 |
a) OD: optical density. Each value represents the mean±S.E. of 3 experiments. Significantly different from control group, ** p<0.01.
The effects of the same fractions on MMP-1 secretion from IL1-β-stimulated HGFs were assessed. Results of Western blotting analysis are shown in Figs. 2 to 5. As shown in Fig. 2, both EtOAc- and water-soluble fractions at a concentration of 10 µg/mL significantly inhibited MMP-1 secretion at 48 h compared with the vehicle control group. The EtOAc-soluble fraction showed more potent inhibition than the water-soluble fraction, but it had no cytotoxic effect (data not shown). As shown in Fig. 3, among five column chromatographic fractions (Frs. A to E), Fr. C at a concentration of 5 µg/mL had the most potent inhibitory activity without cytotoxic effects (data not shown). As shown in Fig. 4, assay results of three fractions (Frs. C-1, C-2, and C-3) obtained from Fr. C by preparative TLC indicated that Fr. C-2 exhibited the most potent activity at 2.5 and 5 µg/mL without cytotoxic effects (data not shown), whereas Frs. C-1 and C-3 were inactive at 2.5 and 5 µg/mL. Two known unsaturated fatty acid amides, (2E,4E,8Z,10E)-N-(2-methylpropyl)dodeca-2,4,8,10-tetraenamide (1) and (2E,4E,8Z,10Z)-N-(2-methylpropyl)dodeca-2,4,8,10-tetraenamide (2), were isolated by preparative HPLC and identified as active constituents of Fr. C-2. As shown in Figs. 5Aa and Ab, 1 showed MMP-1 secretion inhibitory activity at 20 and 40 µM, whereas 2 showed dose-dependent activity at 10, 20, and 40 µM. These two amides lacked cytotoxic effects at 10, 20, and 40 µM, as illustrated in Fig. 6.

Fibroblasts were exposed to IL-1β and then cultured in serum-free medium containing test samples for 48 h. MMP-1 protein levels in the culture medium were assessed by Western blotting analysis using an antibody against human MMP-1. The blot is representative of three separate experiments and represents a single immunoblot.

Fibroblasts were exposed to IL-1β and then cultured in serum-free medium containing test samples for 48 h. MMP-1 protein levels in the culture medium were assessed by Western blotting analysis using an antibody against human MMP-1. The blot is representative of three separate experiments and represents a single immunoblot.

Fibroblasts were exposed to IL-1β and then cultured in serum-free medium containing test samples for 48 h. MMP-1 protein levels in the culture medium were assessed by Western blotting analysis using an antibody against human MMP-1. The blot is representative of three separate experiments and represents a single immunoblot.

(A), (B) Fibroblasts were exposed to IL-1β and then cultured in serum-free medium containing test samples for 48 h. MMP-1 protein levels in the culture medium were assessed by Western blotting analysis using an antibody against human MMP-1. (a) The blot is representative of three separate experiments and represents a single immunoblot. (b) All data are reported as the mean±S.E. of three separate experiments. Significantly different from the vehicle control group, ** p<0.01.
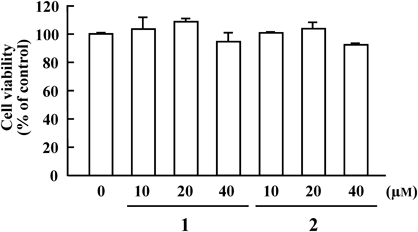
Fibroblasts were incubated in serum-free culture medium in the presence of the indicated concentration of samples for 48 h. Cell viability was determined using a WST-8 assay. All data are given as the mean±S.E. of three separate experiments.
The activation of MAPKs after the addition of IL-1β was preliminarily examined by Western blotting analysis. The time–courses of JNK and p38 phosphorylation levels are shown in Fig. 7A. IL-1β stimulation resulted in a rapid and transient activation of JNK phosphorylation at 0.5 h after stimulation. p38 phosphorylation levels were also remarkably enhanced in a rapid and transient manner at 0.5 h after stimulation. The basal levels of JNK and p38 in HGFs were not affected by IL-1β stimulation.

(A) Fibroblasts were exposed to IL-1β and then cultured in the serum-free medium for the indicated time periods. Total cellular proteins were prepared for Western blotting analysis of MAPKs and phosphor-MAPKs proteins using antibodies against the phospho-forms and total-forms of JNK and p38. β-Actin was used as an internal control. The blot is representative of three separate experiments and represents a single immunoblot. (B) Fibroblasts were exposed to IL-1β and then cultured in serum-free medium for 0.5 h. Total cellular proteins were prepared for Western blotting analysis of MAPKs and phosphor-MAPKs proteins using antibodies against the phospho-forms and total-forms of JNK and p38. β-Actin was used as an internal control. The blot is representative of three separate experiments and represents a single immunoblot.
The effect of 2 (10, 20, 40 µM) on JNK and p38 phosphorylation in IL-1β-stimulated HGFs was examined. On the basis of the preliminary results above (Fig. 7A), phosphorylation levels were assessed at 0.5 h after IL-1β-stimulation. As shown in Fig. 7B, 2 (10, 20, 40 µM) obviously inhibited the phosphorylation of both JNK and p38 at 0.5 h after stimulation.
IL-1β-Stimulated MMP-1 Secretion Inhibitory Activity of Synthetic Analogues of 2Some analogues (3, 4, 5; Fig. 9) of 2 were synthesized and examined for their IL-1β-stimulated MMP-1 secretion inhibitory activities. As shown in Fig. 10, the order of inhibitory activity at a concentration of 20 µM was as follows; 4 with single double bond, 2 and 3 with two double bonds, and a saturated analogue (5) without a double bond.



Fibroblasts were exposed to IL-1β and then cultured in serum-free medium containing test samples for 48 h. MMP-1 protein levels in the culture medium were assessed by Western blotting analysis using an antibody against human MMP-1. The blot is representative of three separate experiments and represents a single immunoblotting.
During the course of our literature search on traditional Chinese medicine, we found that Asiasarum root has been frequently used by itself and as a component in traditional Chinese medicinal (Kampo) formulas for the management of oral malodor syndrome caused by periodontal disease.2) For example, in an ancient traditional Chinese medicinal book: Beiji qianjin yaofang, ed. by Sun Simiao at the middle of 7th century, an aqueous decoction of Asiasarum root was used as a gargle for the treatment of oral malodor syndrome.2) In addition, several traditional Chinese medicinal formulas containing Asiasarum root were used as gargles or internal medicines for the treatment of oral malodor syndrome caused by periodontal disease.2) Among them, a Kampo formula (Rikkosan) has been applied to the treatment of the pain of tooth and/or gingiva. It has been reported that oral administration of Rikkosan after gargling with Rikkosan was more effective for treatment of pain after tooth extraction and glossodynia.30,31) These descriptions may imply that a part of Asiasarum root decoction could penetrate into periodontal tissue when it was used as gargles for the treatment of periodontal disease. However, there are no scientific reports on Asiasarum root related to the inhibitory effects of oral malodor syndrome and periodontal disease or the mechanism of action, and no scientific pharmacokinetic study. The objective of this study was to examine the inhibitory effects of Asiasarum root and its constituents on oral malodor syndrome and periodontal disease.
AR-ext had no antibacterial activity against P. gingivalis. However, AR-ext showed significant METase inhibitory activity at a concentration of 200 µg/mL. In order to identify the active constituents, activity-guided fractionation was carried out by solvent extraction and several chromatographic separations. Repeated chromatography of the EtOAc-soluble fraction, which showed significant activity in the assay, afforded an active oil that showed a single spot on TLC. NMR spectral data suggested that the active oil was a mixture of several fatty acids, and by GC-MS analysis after methylation, the oil was found to be comprised of linoleic acid, palmitic acid, and oleic acid. Authentic compounds of these three detected fatty acids were assayed to find which component was responsible for the inhibition. As shown in Table 2, linoleic acid and oleic acid, major unsaturated acid components of the oil, dose dependently showed a weak activity at 10 to 35 µM in comparison with saturated palmitic acid, but the IC50 value of each acid could not be determined owing to their low solubility in the assay solution. The METase inhibitory activity of these fatty acids has not been reported previously. As a result, it was assumed that the METase inhibitory activity of AR-ext was attributable to these unsaturated fatty acids. As a METase inhibitor from natural sources, Myrsinoic acid B was isolated from Myrsine seguinii.25) It was reported that Myrsinoic acid B inhibited crude methioninase from periodontal bacteria such as Fusobacterium nucleatum, P. gingivalis, and Treponema denticola and the IC50 value were 10.5, 82.4, and 30.3 µM respectively.25) Linoleic acid and oleic acid are not specific constituents of Asiasarum root. However, considering the content of these fatty acids in AR-ext, it was assumed that the METase inhibitory activity of AR-ext is attributable to these fatty acids. Isolation of other active constituents from Asiasarum root is in progress.
As shown in Fig. 1, AR-ext inhibited IL-1β-stimulated MMP-1 secretion from HGFs at a concentration of 10 and 50 µg/mL without cytotoxic effects. The culture time of HGFs was set at 48 h, because the maximum MMP-1 secretion was observed at 48 h after IL1-β-stimulation.
Similar activity-guided fractionation of AR-ext was carried out using MMP-1 secretion inhibitory activity assay to identify active constituents. The effect of the each fraction described above on MMP-1 secretion from IL-1β-stimulated HGFs was assayed. As shown in Fig. 2, the EtOAc- and water-soluble fractions at a concentration of 10 µg/mL significantly inhibited MMP-1 secretion at 48 h compared with the vehicle control group. The EtOAc-soluble fraction showed more potent inhibition than the water-soluble fraction, but had no cytotoxic effect. Fraction C of the EtOAc-soluble fraction at a concentration of 5 µg/mL showed the most potent inhibition without cytotoxic effect. Among three fractions (Frs. C-1, C-2, and C-3) obtained from Fr. C, Fr. C-2 showed the most potent activity at 2.5 and 5 µg/mL without cytotoxic effects, whereas Frs. C-1 and C-3 were inactive at 5 µg/mL. Unsaturated fatty acid amides 126) and 226) (Fig. 8) were isolated and identified as active constituents of Fr. C-2 and lacked cytotoxic effects. As shown in Figs. 5Aa and Ab, MMP-1 secretion inhibitory activity of 2 with the 10-Z configuration was superior to that of 1 with the 10-E configuration. Amides (1 and 2) have been isolated as pungent principles of Asiasarum root.32) It has been reported that 1 has inhibitory activities on lipopolysaccharide (LPS)-induced NO production,33) prostaglandin E(2) production in RAW264.7 mouse macrophage cells,34) and cyclooxygenase (COX)-235); however, the MMP-1 secretion inhibitory activity of these amides was found for the first time. In addition, 1 and 2 had no METase inhibitory activity.
It has been reported that myricetin (1 µM) inhibited lipopolysaccharide-activated mRNA expression of MMPs in HGFs15) and commercial 80% theaflavins (100 µg/mL) inhibited the secretion and mRNA expression of MMP-1 by P. gingivalis-stimulated HGFs.16) Thus we considered that the tested concentration of AR-ext and the amides in our experiments is reasonable.
IL-1β induces an increase in the phosphorylation of JNK and p38 MAPK in HGFs, via activation of the p38 MAPK cascade,13,14) and also induces an increase in the expression and release of MMP-1 via increased c-Jun and c-Fos expression with activation of activator protein-1 (AP-1).13) We examined the effect of IL-1β on the activation of MAPKs in cultured HGFs. Sudden transient phosphorylation of JNK and p38 MAPK was observed at 30 min after IL-1β stimulation. In fact, it has been reported that p38 MAPK plays an important role in MMP-1 production,13,14) and that phosphorylation of ERK also participates in MMP-1 production.13) In our experiments using cultured HGFs, there was no difference in the phosphorylation level of extracellular signal-regulated kinase (ERK) with or without IL-1β stimulation (data not shown). These results indicated that the effect of phosphorylation of JNK and p38 MAPK on increased MMP-1 production in HGFs was superior to that of phosphorylation of ERK in our experimental conditions.
To elucidate the inhibitory mechanisms of 2 on MMP-1 secretion, we investigated its effect on the phosphorylation of JNK and p38 MAPK. Western blotting analysis revealed that 2 reduced the phosphorylation of JNK and p38 MAPK at concentrations of 10, 20, and 40 µM. These results suggested that 2 suppresses intracellular MMP-1 expression and MMP-1 secretion from IL-1β-stimulated HGFs by down-regulation of the phosphorylation of JNK and p38 MAPK.
As an attempt at structure–activity relationship analysis, three analogues (3, 4, and 5) of 2 were synthesized and assayed for their inhibitory activity on MMP-1 secretion. It was found that 2,4-dien amide (3) and 2,4,8,10-tetraen amide (2) had similar activities. Analogue (4) with a single double-bond at the 2 position exhibited the most potent activity, but a saturated analogue (5) showed poor activity. These results suggested that the double bond at the 2 position of 2 may be an essential part of the structure required for the inhibitory activity.
These results suggested that Asiasarum root may be useful for the management of oral malodor syndrome and periodontal disease. However, further scientific pharmacokinetic study on Asiasarum root and its constituents is desirable.
AR-ext showed METase inhibitory activity and inhibited IL-1β-stimulated MMP-1 secretion from HGFs without cytotoxic effects. Activity-guided fractionation of AR-ext suggested that METase inhibitory activity was attributable to a mixture of linoleic and oleic acid, and MMP-1 secretion inhibitory activity were attributable to two unsaturated fatty acid amides, 1 and 2. These findings explain one aspect of the traditional pharmacological efficacy of Asiasarum root, and the root may be useful for the management of oral malodor syndrome and periodontal disease.
The authors are grateful to Mr. Shin-ichi Matsumura, Inabata Koryo Co., Ltd. for GC-MS measurements. This work was supported in part by the Kindai University Fund for Antiaging Center Project.
The authors declare no conflict of interest.