2016 Volume 39 Issue 5 Pages 887-890
2016 Volume 39 Issue 5 Pages 887-890
Hydrogen sulfide (H2S), the third gasotransmitter, is endogenously generated by certain H2S synthesizing enzymes, including cystathionine-γ-lyase (CSE) and cystathionine-β-synthase (CBS) from L-cysteine in the mammalian body. Several studies have shown that endogenous and exogenous H2S affects the proliferation of cancer cells, although the effects of H2S appear to vary with cell type, being either promotive or suppressive. In the present study, we determined whether endogenously formed H2S regulates proliferation in human gastric cancer AGS cells. CSE, but not CBS, was expressed in AGS cells. CSE inhibitors, DL-propargylglycine (PPG) and β-cyano-L-alanine (BCA), significantly suppressed the proliferation of AGS cells in a concentration-dependent manner. CSE inhibitors did not increase lactate dehydrogenase (LDH) release in the same concentration range. The inhibitory effects of PPG and BCA on cell proliferation were reversed by repetitive application of NaHS, a donor of H2S. Interestingly, nuclear condensation and fragmentation were detected in AGS cells treated with PPG or BCA. These results suggest that endogenous H2S produced by CSE may contribute to the proliferation of gastric cancer AGS cells, most probably through anti-apoptotic actions.
Hydrogen sulfide (H2S) is a colorless and highly water-soluble gas with irritant smell like rotten eggs contained in the volcanic gas. Although atmospheric H2S gas is toxic and lethal at concentrations higher than 500 ppm,1) H2S is now considered the third gasotransmitter after nitric oxide (NO) and carbon monoxide (CO).2) In the mammalian tissues, H2S is endogenously synthesized from L-cysteine by enzymes including cystathionine-γ-lyase (CSE), cystathionine-β-synthase (CBS) and 3-mercaptopyruvate sulfurtransferase (3MST) with cysteine aminotransferase (CAT).2,3) It has been reported that endogenous H2S is involved in various physiological/pathophysiological responses, e.g. long-term synaptic potentiation, blood vessel relaxation, cardioprotection, inhibition of platelet aggregation, cell viability change, inflammation, pain sensation, etc.2,4–7) H2S appears to play a dual role in cell viability, being cytoprotective and cytotoxic depending on cell types.2,6,8) In the stomach, however, endogenous and exogenous H2S may be primarily protective against mucosal injury.8–11) We have reported that NaHS, an H2S donor, exhibits a cytoprotective effect against oxidative stress in the gastric mucosa.8) In addition, Wallace et al.11) have reported that endogenous H2S produced by CSE in the gastric mucosa acts to promote ulcer healing. On the other hand, there are inconsistent papers concerning the effects of H2S on gastric cancer cell proliferation and related angiogenesis.12,13) In the present study, we evaluated the role of endogenous H2S in gastric cancer proliferations in human gastric cancer AGS cells. Our results showed that endogenous H2S synthesized by CSE promotes the proliferation of AGS cells, most probably through inhibition of apoptosis.
DL-Propargylglycine (PPG) and β-cyano-L-alanine (BCA) were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.), and NaHS was from Kishida Chemical (Osaka, Japan).
Cell CultureHuman gastric adenocarcinoma AGS cells were purchased from Sumitomo Dainippon Pharma (Osaka, Japan) and cultured in glucose-containing RPMI-1640 (Wako Pure Chemical Industries, Ltd., Osaka, Japan) supplemented with 10% fetal calf serum (FCS; Thermo Electron, Melbourne, Australia), 100 U/mL penicillin and 100 µg/mL streptomycin (Gibco, Carlsbad, CA, U.S.A.) in a 5% CO2 incubator at 37°C.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) AssayAGS cells (2×103 cells/well) were seeded in 96-well plates in the culture medium mentioned above and cultured for one day. Then, CSE inhibitors were added and further cultured for 48 h. The cell number was determined by using a MTT cell proliferation kit (Cayman Chem., Ann Arbor, MI, U.S.A.). NaHS, an H2S donor, at 1.5 mM was repetitively applied at 0, 12, 24 and 36 h after the addition of CSE inhibitors. The data are represented as the percentage of the cell numbers just before the addition of CSE inhibitors.
Lactate Dehydrogenase (LDH) Release AssayForty eight hours after the addition of CSE inhibitors, the activity of LDH released from the cells into the culture medium was measured with an LDH cytotoxicity detection kit (TaKaRa Bio, Otsu, Japan). The activity of released LDH was standardized by the total amount of LDH in the cells, which was obtained by determining the LDH activity from the cells lysed with 1% Triton X-100 (Kishida Chemical). The data are represented as the percentage of the levels of LDH activity just before the addition of CSE inhibitors.
Hoechst 33258 Staining for Detection of ApoptosisAGS cells (1.5×105 cells) were seeded in 6-well plates and cultured for one day. After 48-h incubation with CSE inhibitors, cells were harvested with a cell scraper, fixed in phosphate buffered saline (PBS) containing 1% glutaraldehyde for 30 min at room temperature, and then, stained with Hoechst 33258 (Wako Pure Chemical Industries, Ltd.). A drop of the cell suspension was placed on a slide glass and covered with a coverslip. The nuclear condensation and fragmentation of the cells were morphologically evaluated under UV excitation light with an inverted fluorescence microscope (BX50; Olympus, Tokyo, Japan). The cells with nuclear condensation and fragmentation were counted, and the results are expressed as the percentage of total cells (the proportion of apoptotic cells).
Western BlottingAGS cells (1.2×106 cells/dish) were seeded in 100 mm culture dishes and cultured for one day. The cells were lysed in sodium dodecyl sulfate (SDS) buffer (2% SDS, 62.5 mM Tris–HCl and 10% glycerol, pH 6.8). The protein sample was separated by electrophoresis on 12.5% SDS-polyacrylamide gels (Wako Pure Chemical Industries, Ltd.), and transferred onto polyvinylidene difluoride membrane (Millipore, Bedford, MA, U.S.A.). Primary antibodies used in the present study were: rabbit anti-CSE polyclonal antibody (Sigma-Genosys/Sigma-Aldrich) against a peptide corresponding to the amino acid sequence, (C)80GGTNRYFRR89V, in rat CSE,14) mouse CBS monoclonal antibody (clone 3E1; Abnova Co., Taipei, Taiwan) and rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) polyclonal antibody (sc-25778; Santa Cruz Biotechnol., Santa Cruz, CA, U.S.A.). We used the polyclonal antibody against rat CSE in the present study, because we have accumulated evidence that this antibody specifically recognizes not only rat and mouse CSE,15,16) but also human CSE.17) On the other hand, we used the monoclonal antibody against human CBS, the specificity of which was confirmed in our recent study.17) After washing the primary antibodies, the membrane was then incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse antibodies (Cell Signaling Technol., Beverly, MA, U.S.A.). Immunolabelled proteins were visualized by Chemi-Lumi One Super (Nacalai Tesque, Kyoto, Japan). The protein sample of mouse liver homogenate was used as positive control for CSE and CBS.
StatisticsData are represented as mean±standard error of the mean (S.E.M.). Statistical significance was evaluated by ANOVA followed by Tukey’s test. Significance was set at a level of p<0.05.
Protein expression of CSE, but not CBS, was detected in AGS cells (Fig. 1A). Cell proliferation of AGS cells for 48 h was suppressed by CSE inhibitors, DL-propargylglycine (PPG) and β-cyano-L-alanine (BCA), in a concentration-dependent manner (Figs. 1B, C). The suppressive effects of PPG and BCA were significant 0.5–5 and 0.95–5 mM, respectively. CSE inhibitors in the same range of the concentrations did not increase LDH release from the cells (Figs. 1D, E), indicating that these CSE inhibitors suppressed the cell proliferation without causing cell death. To determine whether endogenous H2S synthesized by CSE actually contributes to the proliferation of AGS cells, effects of NaHS, an H2S donor, on the inhibitory effects of PPG and BCA on proliferation were examined. Repetitive application of NaHS significantly reversed the suppression of proliferation by PPG and BCA (Fig. 2). Both PPG and BCA significantly increased proportion of apoptotic cells with nuclear condensation and fragmentation, as assessed by the Hoechst 33258 staining (Fig. 3).
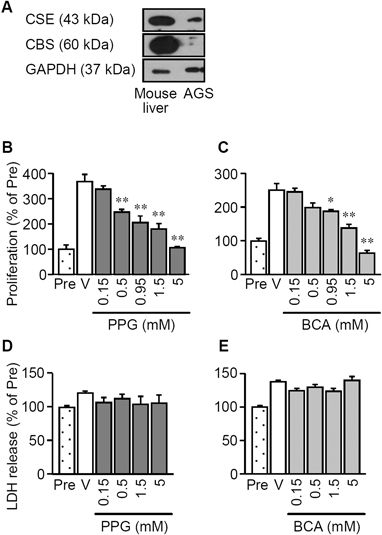
(A) Detection of protein expression of CSE, but not cystathionine-β-synthase (CBS), in AGS cells. Mouse liver is used as a positive control. (B–E) Cell proliferation and cell damage assessed by the MTT method (B, C) and released LDH activity (D, E), respectively, were determined 48 h after addition of CSE inhibitors. Note that both CSE inhibitors suppressed cell proliferation (B, C) without cell damage (D, E). Data show the mean±S.E.M. from 8 (B, C) or 4 (D, E) experiments. * p<0.05, ** p<0.01, vs. vehicle (V).

NaHS at 1.5 mM was repetitively added 0, 12, 24 and 36 h to the cells after addition of CSE inhibitors. Cell proliferation was determined 48 h after addition of the CSE inhibitors. Data show the mean±S.E.M. from 16 (A), 16–24 (B) experiments. ** p<0.01 vs. V+V. † p<0.05, †† p<0.01 vs. V+PPG or BCA. V, vehicle; PPG, DL-propargylglycine; BCA, β-cyano-L-alanine.
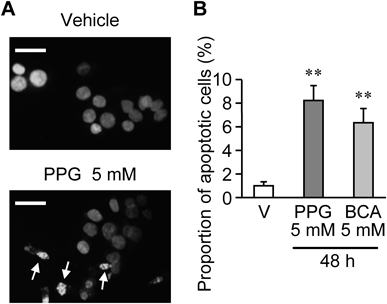
(A) Arrows indicate cells with nuclear condensation and fragmentation, namely apoptotic cells. Scale bar; 50 µm. (B) Data show the mean±S.E.M. from 4 experiments. ** p<0.01 vs. V. PPG, DL-propargylglycine; BCA, β-cyano-L-alanine; V, vehicle.
Our data suggest that the proliferation of human gastric cancer AGS cells is enhanced by the endogenous H2S synthesized by CSE, which exhibits anti-apoptotic activity. CBS is not considered responsible for H2S production in AGS cells, since the protein expression of CBS was hardly detected in AGS cells (Fig. 1A). It has been reported that the increased H2S production plays a crucial role in cell proliferation and the related angiogenesis in several types of cancer cell lines, such as colonic and ovarian cancers.18,19) In addition, there are many papers showing that endogenous or exogenous H2S modulates cell proliferation and migration in gastric, oral, breast, pancreas, lung or prostate cancer and also leukemia,12,20–22) whereas, H2S promotes and suppresses cancer growth in distinct cells. Thus, the effects of H2S on cancers might vary with the tissues, conditions surrounding cancer, the amount of synthesized H2S, and so on.
Our data suggest the involvement of anti-apoptotic effect of H2S in the enhancement of cell proliferation via the CSE/H2S pathway (Fig. 3). It has been reported that H2S enhances the activity of nuclear factor kappaB (NF-κB) through the sulfhydration of the p65 subunit, leading to anti-apoptotic transcriptional activity.23) The H2S/NF-κB/anti-apoptotic pathway might contribute to the H2S-mediated increase in proliferation of AGS cells. On the other hand, our group has reported that H2S enhances the function of Cav3.2 T-type calcium channels.5,24,25) In addition, an independent group has reported the contribution of Cav3.2 to the cell proliferation of prostate cancer LNCaP cells.26) However, the H2S/Cav3.2 pathway is not considered to be involved in the H2S-mediated proliferation of AGS cells, because we could not detect the T-type calcium channel-dependent currents in AGS cells by the whole cell patch clamp technique (data not shown). Further studies are needed to clarify the target molecules of H2S for the H2S-mediated proliferation in future.
This research was supported in part by Grant-in-Aid for Scientific Research (C) Number 23590122 from Japan Society for the Promotion of Science, 2011–2013, and also by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2014–2018 (S1411037).
The authors declare no conflict of interest.