2016 Volume 39 Issue 6 Pages 977-983
2016 Volume 39 Issue 6 Pages 977-983
In the development of therapeutic approaches for central nervous system diseases, a significant obstacle is efficient drug delivery across the blood–brain barrier owing to its low permeability. Various nanocarriers have been developed for brain-targeted drug delivery by modification with specific ligands. We have previously developed polyethylene glycol-modified liposomes (Bubble liposomes [BLs]) that entrap ultrasound (US) contrast gas and can serve as both plasmid DNA or small interfering RNA carriers and US contrast agents. In this study, we attempted to prepare brain-targeting BLs modified with Angiopep-2 (Ang2) peptide (Ang2-BLs). Ang2 is expected to be a useful ligand for the efficient delivery of nanocarriers to the brain. We showed that Ang2-BLs interacted specifically with brain endothelial cells via low-density lipoprotein receptor-related protein-1. We also confirmed that Ang2-BLs could entrap US contrast gas and had US imaging ability as well as unmodified BLs. Furthermore, we demonstrated that Ang2-BLs accumulated in brain tissue after intravascular injection. These results suggested that Ang2-BLs may be a useful tool for brain-targeted delivery and US imaging via systemic administration.
To treat central nervous system diseases, such as malignant brain tumors, neurodegenerative disorders, brain infections, and brain injury, the main obstacle is the delivery of therapeutic drugs across the blood–brain barrier (BBB) owing to its low permeability. Recently, invasive and noninvasive approaches for brain-targeting drug delivery have been investigated. Direct injection methods, such as intraparenchymal, intrathecal, and intracerebroventricular injection, are effective, but they only result in localized delivery, and they can easily bring about infection. As noninvasive methods, nanocarriers have been employed to deliver diagnostic and therapeutic agents to the central nervous system, and various mechanisms have been identified or proposed to explain the transport of nanocarriers to the brain.1,2) As a way to deliver nanocarriers efficiently to the brain, Angiopep-2 (Ang2) peptide is expected to be a useful ligand. Ang2 was discovered through the screening of a designed peptide library based on the Kunitz protease inhibitor domain of aprotinin and shown to display high transcytosis capacity and parenchymal accumulation via low-density lipoprotein receptor-related protein-1 (LRP1).3,4) Ang2 has also been investigated for its ability to deliver drugs, genes, and peptides to the brain.5–9)
We previously developed Bubble liposomes (BLs). These liposomes are polyethylene glycol (PEG)-modified liposomes that contain echo-contrast gas, which can function as a plasmid DNA (pDNA) and small interfering RNA (siRNA) delivery tool and ultrasound (US) imaging tool when used with US exposure in vitro and in vivo.10–14) We have successfully developed targeted BLs modified with peptides and showed the specific attachment, gene delivery, and US imaging ability for tumor neovessels in vitro and in vivo.15,16) We assumed that the modification of BLs with Ang2 could be useful for brain-targeted delivery and US imaging. In this study, we attempted to prepare brain-targeted BLs using Ang2. Furthermore, we assessed the capabilities of BLs for attachment to brain endothelial cells and accumulation in brain tissues.
To prepare liposomes for BLs, 1,2-distearoylphosphatidylcholine (DSPC), 1,2-distearoylphosphatidylethanolamine–methoxy–PEG (PEG2000 or PEG750), and 1,2-distearoylphosphatidylethanolamine–PEG–maleimide (PEG2000-Mal) were used. DSPC, PEG2000, and PEG2000-Mal were purchased from NOF Corporation (Tokyo, Japan), and PEG750 was purchased from Avanti Polar Lipids (Alabaster, AL). As a sizing filter, 200-nm polycarbonate membrane was purchased from ADVANTEC Toyo Roshi Kaisha, Ltd. (Tokyo, Japan). Perfluoropropane gas was obtained from Takachiho Chemical Inc., Co., Ltd. (Tokyo, Japan). Angiopep-2-Cys peptide (Ang2: TFFYGGSRGKRNNFKTEEY-GGC) and Angiopep-7-Cys peptide (Ang7: TFFYGGSRGRRNNFRTEEY-GGC) were custom synthesized (Life Technologies, Inc.). For the competition assay, an anti-LRP1 antibody (Abcam, Cambridge, MA, U.S.A.) was used.
Preparation of LiposomesLiposomes were prepared using the reverse phase evaporation method as described previously.13) Briefly, DSPC and PEG2000 or PEG750 were mixed at a molar ratio of 92 : 2, and dissolved in 1 : 1 (v/v) chloroform–diisopropylether. N-(2-Hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES)-buffered saline (HBS; 150 mM NaCl, 10 mM HEPES, pH 7.0) was added to the lipid solution, and the mixture was sonicated and then evaporated. The organic solvent was removed completely, and the size of the liposomes was adjusted to less than 200 nm using extrusion equipment and a sizing filter. After sizing, the liposomes were filter-sterilized using a 0.45-µm syringe filter (Asahi Techno Glass Co., Chiba, Japan). For fluorescent labeling of the lipid membrane, 1,1-dioctadecyl-3,3,3,3-tetramethyl-indocarbocyanine perchlorate (DiI: 0.1 or 1 mol% of total lipids) was added. The liposome concentration was determined using a phosphorus assay.
Preparation of Peptide-Modified LiposomesPeptide-modified liposomes were prepared by the post-insertion method.17) Dried lipid films containing PEG2000–Mal were hydrated in HBS buffer with gentle agitation and heating at 65°C. An adequate amount of peptide (Ang2 or Ang7) was added to the micelles in the presence of tris(2-carboxyethyl)phosphine hydrochloride, and the mixture was incubated for 6 h at room temperature. To prepare peptide-modified liposomes (Ang2-lipo or Ang7-lipo), 6 mol% of peptide-coupled PEG micelles were mixed with preformed liposomes for 1 h at 60°C. For the preparation of peptide-unmodified liposomes (PEG-lipo), 6 mol% of PEG micelles without peptide were mixed with preformed liposomes for 1 h at 60°C. The resulting liposomes were passed through a Sephadex G-50 spin column to remove any excess peptides. The liposome concentration was determined using a phosphorus assay.
Preparation of BLsBLs were prepared from liposomes and perfluoropropane gas. First, 5-mL sterilized vials containing 2 mL of liposome suspension (total lipid concentration: 1 mg/mL) were filled with perfluoropropane gas, capped, and then pressurized with 7.5 mL of perfluoropropane gas. The vials were placed in a bath sonicator (42 kHz, 100 W; Bransonic 2510J-DTH; Branson Ultrasonics Co., Danbury, CT, U.S.A.) for 5 min to form BLs. The zeta potential and mean size of the BLs were determined using a light scattering method with a zeta potential/particle sizer (Nicomp 380ZLS, Santa Barbara, CA, U.S.A.).
Cell CulturesThe endothelioma cell line bEnd.3, established from mouse brain capillaries, was cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 4.5 g/L glucose (Kohjin Bio Co., Ltd., Tokyo, Japan) and supplemented with 10% heat-inactivated fetal bovine serum (Equitech Bio Inc., Kerrville, TX, U.S.A.), 100 U/mL penicillin, and 100 µg/mL streptomycin in a humidified atmosphere containing 5% CO2 at 37°C.
Cellular Binding and Competition AssayThe day before the experiments, bEnd.3 cells (2.5×104 cells/well) were seeded in a 48-well plate (Asahi Techno Glass Co., Chiba, Japan). Cells were incubated with DiI-labeled liposomes (200 µg (total lipid)) for 1 h at 37°C. To confirm the binding of free peptide to bEnd.3 cells, fluorescein isothiocyanate (FITC)-labeled peptide, which was the equivalent to 6 mol% of 200 µg liposomes, was added to cells. The cells were washed and collected, and then fluorescence intensities were measured by flow cytometry (FACSCanto: BD Biosciences, Franklin Lakes, NJ, U.S.A.).
To examine the specific attachment of BLs, bEnd.3 cells (2.5×104 cells/well) were seeded in a 48-well plate the day before the experiments. DiI-labeled BLs (200 µg (total lipid)) were added to well, and the plate was sealed with sterile tape and inverted for 5 min. The plate was put back for 3 min to remove BLs that did not attach to cells. The cells were washed and collected, and then fluorescence intensities were measured by flow cytometry. For the competition assay, bEnd.3 cells were incubated with anti-LRP1 antibody for 30 min at room temperature before the addition of BLs.
Ultrasound Imaging of BLsBLs diluted with HBS were dispensed into 6-well plates. B-mode recordings were made using a high-frequency ultrasound imaging system (NP60R-UBM, Nepa Gene, Co., Ltd., Chiba, Japan).
Targeting Effect of Ang2-BLs in VivoTo evaluate the targeting effect of Ang2-BLs in vivo, fluorescence imaging analysis was used. Male ICR mice (5 weeks old) were injected with 200 µL of DiI-labeled BLs (PEG-BLs, Ang2-BLs, or Ang7-BLs) via the tail vein. Mice were sacrificed at 10 min post-injection. Major organs (brain, heart, lung, liver, spleen) were dissected, washed with PBS, and subjected to Maestro in vivo imaging system to obtain the fluorescence images. The brain tissues were embedded in Optimal Cutting Temperature (OCT) compound and immediately frozen at −80°C. Serial sections 6 µm thick were cut by cryostat and observed using a fluorescence microscope (BZ8100, KEYENCE, Osaka, Japan).
In Vivo StudiesAnimal use and relevant experimental procedures were approved by the Tokyo University of Pharmacy and Life Sciences Committee on the Care and Use of Laboratory Animals. All experimental protocols for animal studies were in accordance with the Principle of Laboratory Animal Care at Tokyo University of Pharmacy and Life Sciences.
We initially confirmed the binding of free peptide to bEnd.3 cells using an FITC-labeled peptide. The bEnd.3 cell-line has been used as a model of the BBB owing to their rapid growth and maintenance of BBB characteristics over repeated passages. It has also been reported that the LRP1 expression levels on bEnd.3 cells were considerably high.18–21) As a result, the percentage of FITC-positive cells was increased by addition of the Ang2 peptide (Fig. 1). In contrast, the fluorescence intensities of cells treated with Ang7 were similar to those of untreated cells. Then, we prepared DiI-labeled Ang2-lipo and examined the interaction with bEnd.3 cells by flow cytometry. However, the interaction was barely detectable (Fig. 2a). To enhance the interaction of the cells with Ang2 binding to PEG2000–Mal, we changed the maleimide-unmodified PEG chain length from 2000 to 750. As a result, the interaction of Ang2-lipo with bEnd.3 cells increased in a peptide dose-dependent manner (Fig. 2b). Thus, in all subsequent experiments liposomes composed of DSPC, PEG750, and PEG2000-Mal (in a 92 : 2 : 6 M ratio) were used.

bEnd.3 cells were treated with FITC-labeled Ang2 or Angiopep-7 (Ang7; negative control) peptide for 1 h. The fluorescence intensity was measured using a FACSCanto. Gray area: cells only; gray dotted line: Ang7 peptide; black solid line: Ang2 peptide.

bEnd.3 cells were incubated with DiI-labeled Ang2-lipo for 1 h. (a) bEnd.3 cells were incubated with Ang2-lipo (DSPC–PEG2000–PEG2000-Mal=92 : 2 : 6 molar ratio) then analyzed using a FACSCanto. (b) bEnd.3 cells were incubated with Ang2-lipo (DSPC–PEG750–PEG2000-Mal=92 : 2 : 6 molar ratio) and analyzed using a FACSCanto. Gray area: cells only; gray dotted line: PEG-lipo; black solid line: Ang-lipo (4 mol%); black dashed line; Ang-lipo (5 mol%); gray solid line: Ang-lipo (6 mol%).
We attempted to entrap US contrast gas in Ang2-lipo. As shown in Table 1, there was almost no change in the size and zeta potential of BLs in the presence or absence of peptide. To investigate the usability of these BLs as US contrast agents, we used a high-frequency US imaging system. The system is a two-dimensional US image display composed of bright dots representing the US echoes. The brightness of each dot is determined by the amplitude of the returned echo signal. As shown in Fig. 3, the US echo signal was detected even 90 min later, and modification with the Ang2 peptide had no effect on the imaging ability.
| Mean size (nm) | Zeta potential (mV) | |||
|---|---|---|---|---|
| Unmodified | Ang2-modified | Unmodified | Ang2-modified | |
| Liposomes | 133.8±22.4 | 144.7±23.0 | −1.06±1.20 | −1.69±7.2 |
| BLs | 551.4±73.1 | 525.1±59.3 | −0.91±0.80 | −0.88±1.28 |

To examine the specific interaction of Ang2-BLs with bEnd.3 cells, we used Ang7 as a control peptide and an anti-LRP1 antibody. Ang7 was identical to Ang2 except that the lysine residues at positions 10 and 15 were substituted with arginine residues. Ang7 did not exhibit in vitro transcytosis or in vivo accumulation in brain tissue.3,22) The size and zeta potential of Ang7-lipo and Ang7-BLs were similar to those of Ang2-lipo and Ang2-BLs, respectively (data not shown). As shown in Fig. 4, the interaction of Ang2-BLs with bEnd.3 cells was enhanced compared with peptide-unmodified BLs (PEG-BLs) and Ang7-BLs. Furthermore, the interaction was reduced by pretreatment with anti-LRP1. These results suggested that Ang2-BLs interacted with bEnd.3 cells via LRP1.

bEnd.3 cells were treated with DiI-labeled Ang2-BLs or Angiopep-7-modified BLs (Ang7-BLs) (6 mol%) for 5 min. bEnd.3 cells were incubated with Ang2-BLs (DSPC–PEG750–PEG2000-Mal=92 : 2 : 6 molar ratio) and analyzed using a FACSCanto. For the competition assay, bEnd.3 cells were incubated with an anti-LRP1 antibody for 30 min at room temperature before the addition of BLs. Gray area: cells only; gray dotted line: PEG-BLs; black dashed line: Ang7-BLs; black solid line; Ang2-BLs; gray dashed line: Anti-LRP1 antibody and Ang2-BLs.
We evaluated the accumulation of Ang2-BLs in brain tissue after intravenous administration. As a result, Ang2-BLs showed accumulation in brain tissue, whereas PEG-BLs and Ang7-BLs did not show accumulation (Fig. 5a). The differences were also confirmed from brain tissue sections (Fig. 5b). These results suggested that Ang2-BLs possess the targeting activity to brain tissue and could be a useful tool for the delivery of therapeutic agents to the brain in combination with US exposure.
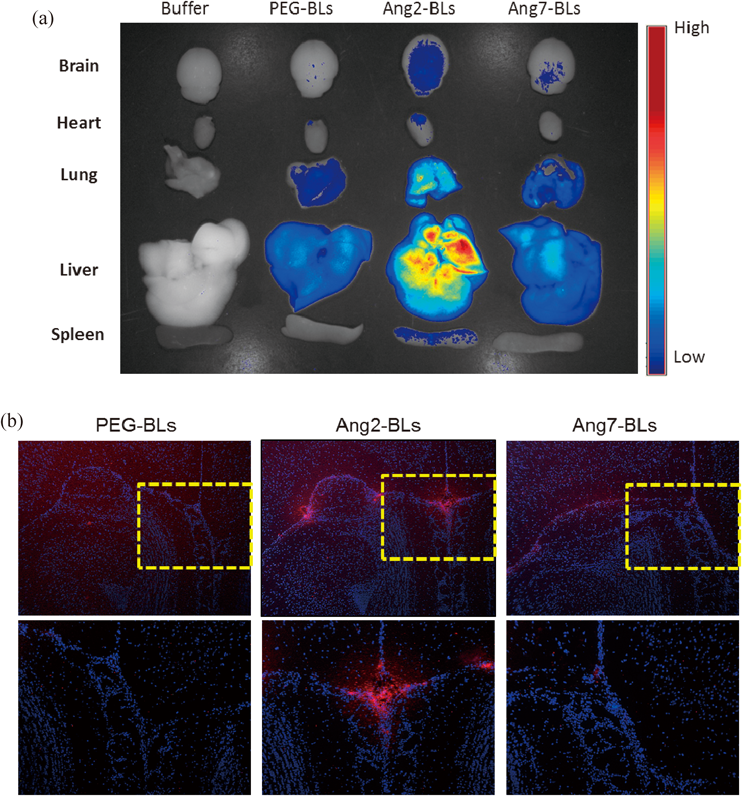
Mice were injected with DiI-labeled Ang2-BLs (200 µg) via the tail vein. (a) Ten minutes after injection, tissues were dissected and analyzed using a Maestro in vivo imaging system. (b) Specimens were embedded in OCT compound and immediately frozen at –80°C. Serial 6-µm thick sections were cut using a cryostat and observed using a fluorescence microscope. Cell nuclei were stained with DAPI. The yellow boxed regions are enlarged at the bottom of each panel.
The term theranostics, which is derived from “diagnostics” and “therapeutics,” refers to a treatment strategy that combines a diagnostic test and specific therapy based on the test results. Recently, various nanoparticles were reported to have potential as theranostic agents for optical imaging, magnetic resonance imaging, nuclear imaging, and computed tomography.23) US imaging is used frequently in clinical practice and has also attracted much attention as a theranostic application. US contrast agents, which are gas-filled microbubbles (MBs) stabilized by a shell made of lipids, proteins, or polymer can enormously improve the effect of US imaging. Furthermore, MBs with US exposure were also reported to show promise in enhancing gene and drug delivery, and targeted MBs were developed using cell-specific ligands, mostly peptides or antibodies, for targeted diagnosis and therapy.24–28) However, it has been assumed that these MBs have difficulty penetrating deep into tissues owing to their size of 1–5 µm. Our nanosized BLs are potentially superior carriers for delivery into tissues via microvessels.
In this study, we focused on Ang2 for brain-targeted delivery. Initially, the prepared Ang2-lipo did not interact with bEnd.3 cells. We changed the chain length of PEG not binding peptide from long to short, and as a result, the interaction was enhanced (Fig. 2b). Conformation of PEG chains on the surface of liposomes is dependent on the PEG’s molecular weight and the graft density. At increasing grafting densities, the PEG chains transition from a “mushroom” conformation to a more extended “brush” conformation. During a transition between the two models, the fixed aqueous layer thickness and PEG flexibility increase.29–31) As a result, it has been suggested that PEG-liposomes have a long circulation time. It has also been reported that the modification of liposomes with short and long PEG chains increases the fixed aqueous layer thickness and improves the circulation in the blood.32) It is suggested that the conformation of long PEG chains is extended by the presence of short PEG chains. Based on these findings, we conclude that the conformation of PEG2000-Mal binding peptide was extended by the presence of PEG750 and resulted in facilitating the interaction between Ang2 and bEnd.3 cells.
It was shown previously that Ang2 possesses high transcytosis capability in both an in vitro model of the BBB and by in situ brain perfusion in mice.3,4) Ang2-modified nanoparticles also displayed this ability in an in vitro model of the BBB, and these nanoparticles were transported via LRP.5,7) Our results showed that Ang2-BLs bound to bEnd.3 cells via LRP1 (Fig. 4). We also confirmed the accumulation of Ang2-BLs in brain tissue after systemic administration (Fig. 5). However, the cell types colocalized with Ang2-BLs were not evaluated in vivo. Further investigations into whether Ang2-BLs possess transcytosis capability might be required, although we assume that it may be difficult for Ang2-BLs to be transported through the BBB; because the particle size of Ang2-modified nanoparticles showed that the transcytosis capability was smaller (100–130 nm) than that of Ang2-BLs. However, we hypothesize that transcytosis capability is not necessarily required for Ang2-BLs to be effective carriers. In future, we will attempt to apply Ang2-BLs to a brain-targeted gene delivery tools in combination with therapeutic US. Recently, it has been reported that using focused US-mediated MBs enabled a transient BBB opening in a localized brain region.33) Therapeutic US can cause oscillating MBs to undergo a collapse known as inertial cavitation within a short time. Inertial cavitation enables MBs not only to cause transient disruptions in cell membranes but also to increase permeability of the BBB. Furthermore, we reported that the combination of our nanosized BLs with a high-intensity focused US could also serve to increase the permeability of the BBB.34) Therefore, we conclude that it is important for Ang2-BLs to bind and accumulate at the BBB rather than it being transported to the brain parenchyma. We also suggest that Ang2-BLs could provide a brain-targeted gene delivery and US imaging tool in combination with US exposure.
The distribution of Ang2-BLs after systemic administration showed high accumulation not only in the brain but also in the lung and liver (Fig. 5a). This may be due to the high expression level of LRP1 in these tissues.35,36) We assume that the use of US exposure to the brain in combination with Ang2-BLs may lead to an increase in the efficiency of brain delivery and a decrease in the influence on other tissues. However, we should investigate the influence on the liver and lung in future studies. In brain tumors and cerebral ischemia, the expression of LRP1 has been reported to be increased.37,38) Therefore, Ang2-BLs could be useful imaging and gene delivery tools for these diseases.
In this study, we showed that Ang2-BLs interacted specifically with bEnd.3 cells via LRP1. Furthermore, we demonstrated that Ang2-BLs accumulated in brain tissue after intravascular injection. These results suggest that Ang2-BLs may be useful for brain-targeted delivery and US imaging via systemic administration.
We are grateful to Prof. Katsuro Tachibana (Department of Anatomy, School of Medicine, Fukuoka University) for technical advice regarding the induction of cavitation with US and to Mr. Yasuhiko Hayakawa and Mr. Kosho Suzuki (NEPA GENE Co., Ltd.) for technical advice regarding US exposure. This study was supported by a Grant for Industrial Technology Research (04A05010) from the New Energy and Industrial Technology Development Organization (NEDO) of Japan, a Grant-in-Aid for Scientific Research (B) (20300179) from the Japan Society for the Promotion of Science, and a Grant-in-Aid for Young Scientists (B) (24790173) from the Japan Society for the Promotion of Science.
The authors declare no conflict of interest.