2016 Volume 39 Issue 6 Pages 903-908
2016 Volume 39 Issue 6 Pages 903-908
Cigarette smoke contains many harmful chemicals that contribute to the pathogenesis of smoking-related diseases such as chronic obstructive pulmonary disease, cancer, and cardiovascular disease. Many studies have been done to identify cytotoxic chemicals in cigarette smoke and elucidate the onset of the above-mentioned diseases caused by smoking. However, definitive mechanisms for cigarette smoke toxicity remain unknown. As candidates for cytotoxic chemicals, we have recently found methyl vinyl ketone (MVK) and acetic anhydride in nicotine/tar-free cigarette smoke extract (CSE) using L-tyrosine (Tyr), an amino acid with highly reactive hydroxyl group. The presence of MVK and acetic anhydride in CSE was confirmed by gas chromatography-mass spectrometry (GC/MS). We also found new reaction products formed in B16–BL6 mouse melanoma (B16–BL6) cells treated with CSE using LC/MS. These were identified as glutathione (GSH) conjugates of α,β-unsaturated carbonyl compounds, MVK, crotonaldehyde (CA), and acrolein (ACR), by the mass value and product ion spectra of these new products. ACR and MVK are type-2 alkenes, which are well known as electron acceptors and form Michael-type adducts to nucleophilic side chain of amino acids on peptides. These α,β-unsaturated carbonyl compounds may have a key role in CSE-induced cell death.
Cigarette smoking is well known as a major risk factor for cardiovascular disease, cancer, and chronic obstructive pulmonary disease.1) Cigarette smoke contains >4800 identified chemical compounds,2) many of which are toxic and harmful to the human body. However, the exact mechanism behind such smoking-related human diseases is not well understood. Increases in reactive oxygen species (ROS) are a well known phenomenon under inflammatory conditions and ROS production is involved in many diseases.3) For the reasons described above, many studies have focused on ROS in cigarette smoke. For example, unstable compounds such as free radical and peroxynitrite,4,5) relatively stable compounds,6,7) and also ROS responsible reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase8) have been examined.
Cigarette smoke includes various α,β-unsaturated carbonyl compounds. Among them, acrolein (ACR) and crotonaldehyde (CA) are two of the most studied compounds. They are suspected candidate factors to trigger generation of O2−.6) α,β-Unsaturated carbonyls selectively form adducts with reactive nucleophilic cysteine residues, which are toxicologically relevant molecular targets.7)
Recently, α,β-unsaturated ketones, methyl vinyl ketone (MVK) and 2-cycopenten-1-one (CPO), were identified by Noya et al.9) in CSE as stable cytotoxic factors. We also identified MVK and other reactive carbonyl compounds in nicotine/tar-free cigarette smoke extract (CSE),10) and further demonstrated that non-enzymatic conjugation of MVK to glutathione (GSH) occurs easily in B16–BL6 mouse melanoma (B16–BL6) cells exposed to CSE.11) In this review, we report recent analytical study about active ingredients in CSE using mass spectrometry.
To discover whether biomolecules can be modified by active ingredients in cigarette smoke, we tried to identify products formed by reaction of CSE with L-tyrosine (Tyr), which contains reactive hydroxyl groups. The reaction products obtained were analyzed by LC/MS and LC/MS/MS. We compared differences in the mass spectra of CSE solution before and after reaction with Tyr solution (Figs. 1a, b). From the mass value of the positive and negative ion mode mass spectra the molecular weight (Mr) of three peaks was presumed 251 for retention times (tR) of 11.2 min (Fig. 1c) and 223 for tR 14.8 and 16.7 min (Fig. 1d). The compounds of Mr 223 (Tyr+42) were estimated as acetylated forms of Tyr because Mr 223 has m/z 42 u higher mass values than Tyr (Mr 181) (Fig. 2). N-Acetyl-Tyr and O-acetyl-Tyr were identified by their respective authentic standards on the basis of the retention time and product ion spectral pattern of their LC/MS chromatograms. Ultimately, the compound of tR 14.8 min was identified as O-acetyl-Tyr and that of tR 16.7 min as N-acetyl-Tyr.

Extracted ion chromatograms of the protonated molecule [M+H]+ at (c) m/z 252 and (d) m/z 224 obtained from CSE solution after reaction with Tyr.

Subsequently, the elemental composition of Mr 251 (Tyr+70) was determined by high-resolution mass spectrometer. The accurately measured mass was 252.1233±0.00048. The difference between the accurately measured mass and the calculated exact mass of C13H18NO4 was −0.00017 u, and the mass error was 0.67 ppm. This indicates that the elementary composition (molecular formula) of the compound Tyr+70 is C13H18NO4.
Gas chromatography-mass spectrometry (GC/MS) analysis was used for identification of active ingredients in CSE that can produce the acetylated compounds Tyr+42 and the compound Tyr+70. Usually GC/MS samples are prepared by derivatization of analyte and extraction into organic solvent. In this study, analysis of active ingredients was accomplished by direct injection of CSE, using ZB-WAX (30 m×0.24 mm i.d.×1.00 µm film thickness, Phenomenex Inc., CA, U.S.A.). The GS/MS analysis data of CSE are shown in Fig. 3a. Two compounds were detected at tR 6.3 and 9.5 min from the mass chromatogram of m/z 70 (Fig. 3b). From library search of their mass spectra, we identified the peak at tR 9.5 min as CA and that at tR 6.3 min as MVK (Fig. 3f). These retention times were the same as those of their respective authentic samples. With regard to compounds Tyr+42, it was confirmed that the acetic acid contained in CSE does not react with Tyr at all, but the acetic anhydride easily reacts with Tyr in Dulbecco’s phosphate-buffered saline (DPBS) (−) solution to produce the acetylated Tyr derivatives N- and O-acetyl-Tyr. This result indicates that acetic anhydride is present in fresh CSE solution and gradually hydrolyzes to acetic acid over time. Thus analytical data for the authentic standard of acetic acid were compared with those of acetic anhydride by GC/MS. Peaks of acetic anhydride and acetic acid were observed at tR 23.3 min on the total ion current (TIC) chromatograms (Figs. 4a, b) and also appeared with the same base peak of m/z 43 in both mass spectra (Figs. 4c, d). From these results, we can surmise that acetic anhydride is easily decomposed to acetic acid by hydrolysis during passage through the GC column. The future challenge is to evaluate whether acetic anhydride adducts with such amino acids to produce carbonyl adducts.

These data indicate that MVK, acetic anhydride, and acetic acid exist in CSE.
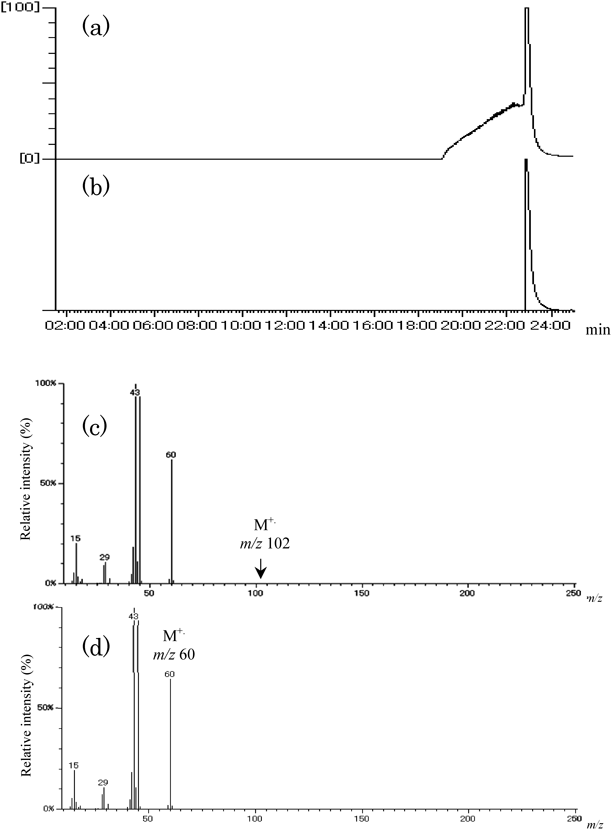
We cannot observe the peak of molecular ion of acetic anhydride (m/z 102) on the mass spectrum of (c). EI mass spectra of these compounds (c, d) are very similar.
We identified MVK and CA as compounds having molecular weight 70 that exist in CSE. To clarify the structure of compound Tyr+70, MVK and CA, components assumed present in CSE, were reacted with Tyr at 37°C. The peak of compound Tyr+70 appeared on the mass spectra of the mixed solution of Tyr and MVK, but was hardly detected on that of the mixed solution of Tyr and CA. This shows that MVK reacts with Tyr to produce compound Tyr+70. Analysis data for compound Tyr+70 synthesized by reaction of MVK with Tyr agreed with those of compound Tyr+70 obtained by reaction of CSE with Tyr. The structure of compound Tyr+70 was identified as N-(3-oxobutyl)-Tyr [3-(4′-hydroxyphenyl)-2-(3″-oxobutylamino)propanoic acid] (Fig. 2) by 1H-, 13C-, and 2D- (HMQC, HMBC) NMR spectra. Kaminskas et al.12) reported that α,β-unsaturated carbonyl compounds, MVK and ACR, react with lysine residues in proteins via their unsaturated bond to form Michael addition adducts, and this reaction is more efficient than Schiff base adduction via their carbonyl group. Those results and this report support that the synthesized N-(3-oxobutyl)-Tyr is obtained by Michael reaction, not by Schiff base reaction of carbonyl compounds with primary amines. Recently, there have been many investigations of α,β-unsaturated carbonyl compound-induced carbonylation.7,13) In CSE, MVK is regarded as one of the important α,β-unsaturated carbonyl compounds because of its reactivity.
To investigate the molecular mechanism of cytotoxicity induced by CSE, B16–BL6 cells were exposed to CSE under conditions that have little effect on viability. In this study, we chose B16–BL6 cells since this cell line is black and highly metastatic.14) In the next step, we are planning to investigate the effect of CSE and its ingredients on the metastatic ability of the cells, since this cell line is easily detectable in secondary metastasized organs in in vivo studies. Cells were exposed to 1% CSE at 37°C for 30 min then deproteinized with 70% methanol/DPBS (−) solution. After ultracentrifuging, the supernatants were directly analyzed by LC/MS.
Several new peaks were observed in the positive ion mode total ion current chromatogram of the sample obtained from cells exposed to CSE (Figs. 5a, b). The new peak at retention time of 16.1 min corresponded to the protonated molecule [M+H]+ at m/z 378 (Fig. 5d). On the other hand, in the mass spectrum of the round peak at retention time of 11–12 min, which appears in Figs. 5c and d, the protonated molecule [M+H]+ at m/z 308 was observed as the base peak. The peak at m/z 308 is thought to correspond to the protonated molecule of GSH ([GSH+H]+) because it is well known that GSH, a major antioxidant, is present in high concentrations in cells (approximately 5 mM). Indeed, we confirmed that the protonated molecule [M+H]+ at m/z 308 detected in the cells corresponds to the protonated molecule of GSH by showing that the product ion spectrum of the ion at m/z 308 matched that of authentic GSH.

The new peak of protonated molecule [M+H]+ at m/z 378 was observed in the cells exposed to CSE.
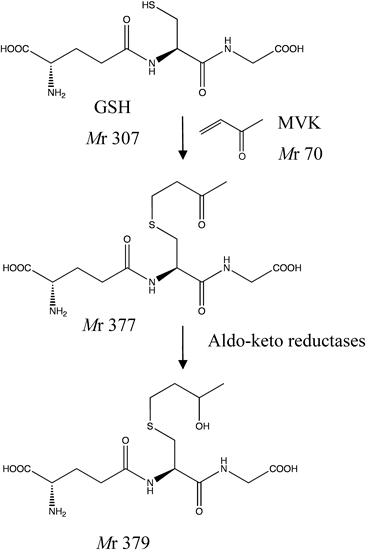
Also, we estimated the mass value m/z 70, which was subtracted m/z 308 from m/z 378, to be two α,β-unsaturated carbonyl compounds, CA and MVK (Mr 70 each), because these compounds are abundant in CSE.
Other investigators have reported similar results.9,15) van der Toorn et al.16) recently demonstrated by MS analysis that a substantial amount of GSH in epithelial cells exposed to the gaseous phase of cigarette smoke is irreversibly modified to GSH-CA derivatives, thereby depleting the total available GSH pool. Moreover, Reddy et al.17) reported that the GSH-CA adduct is a major reaction product of the gas vapor phase of cigarette smoke with GSH. However, these reports do not mention formation of the GSH-MVK adduct. In the present study, we also found that the peak area of m/z 308 (the protonated molecule of GSH) in B16–Bl6 cells treated with 1% CSE for only 5 min dramatically decreases to almost 40% of control. Therefore it seems reasonable that α,β-unsaturated carbonyl compounds in CSE can readily produce GSH adducts via Michael addition. As for MVK, it has been reported that this molecule induces apoptosis in neuronal cells through GSH depletion.18) In addition, GSH reacts with MVK to form the inactive GSH-MVK adduct.19) However, there is no report that exposure of CSE or cigarette smoke to cells results in formation of GSH-MVK adducts. We therefore attempted to react CSE directly with GSH to identify the GSH-carbonyl adducts formed in B16–BL6 cells exposed to CSE.
To clarify whether the ion at m/z 378 detected in cells exposed to CSE is either the GSH adduct with CA or MVK, GSH (1 mM) was reacted with CA and MVK (10 µM each) at 37°C for 30 min. As a result, a peak at m/z 378 corresponding to CA and MVK appeared at tR of 17.1 and 16.1 min, respectively. When GSH was incubated with 1% CSE at 37°C for 30 min, the peak at m/z 378 was observed at a tR of 16.1 min. These findings indicate that the peak at m/z 378 derives from the GSH-MVK adduct. Furthermore, in LC-MS/MS analysis, the product ion spectra of the ion at m/z 378 detected in CSE-exposed cells accorded well with the spectra of the GSH-MVK adduct rather than the GSH-CA adduct. Taken together, these results demonstrate that the protonated molecule [M+H]+ at m/z 378 detected in CSE-exposed cells is mostly derived from GSH-MVK. It seems that MVK in CSE reacts more rapidly with GSH in comparison with CA in the cells and thereby mainly forms the GSH-MVK adduct. Yarbrough and Schultz20) reported that aliphatic α,β-unsaturated carbonyls react with GSH by way of Michael-type nucleophilic addition and that among the homologs, derivatives with a terminally located olefin (such as MVK) are more reactive and more toxic than those with an internally located olefin (such as CA). The structure of GSH-conjugated MVK is shown in Fig. 6, which was elucidated by LC/MS/MS.
Cytotoxic effects of CSE have been reported in various cells.21,22) We clarified that CSE reduces cell viability in a concentration-dependent manner in B16–BL6 cells. In the presence of 1% CSE, cell viability was significantly reduced to about 45.3% at 24 h of incubation. Also, α,β-unsaturated carbonyl compounds, CA and MVK, included in CSE reduced cell viability in the same manner as CSE. At a concentration of 30 µM, CA and MVK reduced cell viability to 54.2% and about 8.4%, respectively. These data suggest that the active ingredients in CSE noticeably affect CSE-induced cytotoxicity. It seems that MVK has a stronger effect on the mechanism of cytotoxicity than CA. Furthermore, the cytotoxicity of CSE and MVK was antagonized significantly by pre-addition of N-acetyl cysteine, a GSH precursor.
In recent years, there has been much interest in elucidation of cytotoxic mechanisms of protein carbonization by ingredients of CSE. However, there is no report on metabolism of α,β-unsaturated carbonyl aldehyde- and ketone-induced carbonylation compounds. We found some information about metabolism of GSH-conjugated α,β-unsaturated carbonyl compounds (aldehyde: CA and ACR and ketone: MVK) using mass spectral analysis of extracted solution of B16–BL6 treated with CSE. These results indicate that these conjugated compounds are reduced to their alcohol forms by aldo–keto reductase23) (Fig. 6). Our findings about the metabolism of GSH-conjugated CA, ACR, and MVK suggest the possibility of using these alcohol forms as biomarkers of oxidative stress in blood after cigarette smoking.
To elucidate the mechanism of smoking-related disease induced by active ingredients in CSE, we have been studying toxic substances similar to ROS. We found new active ingredients, acetic anhydride and MVK, in CSE using reaction activity of amino acid (L-tyrosine) and mass spectral analysis. Our data indicate that MVK plays an important role of oxidative stress because of its high reactivity. In addition, GSH levels in cells were markedly decreased by treatment with CSE. GSH depletion is a common feature of cell death. The depletion of intracellular GSH with the formation of the GSH-MVK adduct may contribute to CSE-induced cytotoxicity.
This study was supported in part by a Grant from the Smoking Research Foundation, Japan.
The authors declare no conflict of interest.