2016 Volume 39 Issue 6 Pages 1055-1059
2016 Volume 39 Issue 6 Pages 1055-1059
Recent reports have shown that dimethyl fumarate (DMF) prevents brain damage induced by intracerebral hemorrhage and this beneficial effect is mediated by the nuclear erythroid 2 p45-related factor-2–antioxidant response element (Nrf2–ARE) pathway. However, the downstream mechanism underlying the activation of the Nrf2–ARE pathway is unclear. Here, we investigated the protective effect of DMF using an in vivo model of oxidative stress induced by sodium nitroprusside (SNP) and rat primary striatal cultures. Oral administration of DMF prevented SNP-induced motor dysfunction. Pre-administration of DMF (60–200 mg/kg) for 24 h dose-dependently protected against brain damage induced by the striatal injection of SNP. Next, we investigated the protective effect and mechanism of DMF against oxidative stress using rat primary striatal cell cultures. Treatment of striatal cells with DMF (10 µM) markedly prevented hydrogen peroxide-induced cytotoxicity. The protective effect of DMF against oxidative stress in vitro was inhibited by zinc protoporphyrin IX, an inhibitor of heme oxygenase-1, but not by buthionine sulfoximine, an inhibitor of glutathione synthesis. These results suggest that the activation of heme oxygenase-1 plays an important role in the protective effect of DMF.
It is suggested that oxidative stress is a risk factor of neuronal death in various brain disorders such as the Alzheimer’s and Parkinson’s diseases.1–3) The nuclear erythroid 2 p45-related factor-2–antioxidant response element (Nrf2–ARE) pathway is known as an endogenous defense mechanism against oxidative stress, and its activation induces the expression of various antioxidant enzymes.4,5) In addition, the substances that activate this pathway have neuroprotective effects; consequently, these substances are considered to be effective for the prophylaxis of neurodegenerative diseases and the slowing of those diseases progress.6,7)
It has been reported that the oral administration of dimethyl fumarate (DMF) in mice activates the Nrf2–ARE pathway in the brain and increases the mRNA expression of reduced nicotinamide adenine dinucleotide phosphate (NAD(P)H): quinone oxidoreductase-1 (NQO1).8) DMF also prevents brain damage induced by intracerebral hemorrhage.9) Furthermore, DMF has been approved for the treatment of multiple sclerosis in many countries. However, the efficacy of DMF for oxidative stress in the brain has not been comprehensively examined.
We previously reported that the microinjection of sodium nitroprusside (SNP) into the striatum induced motor dysfunction and damage in the forebrain, including the striatum and cerebral cortex.10) We utilize this animal model as an in vivo brain oxidative stress model in mice. We previously reported that Fe2+ released from SNP induced oxidative stress and that oxidative stress plays a crucial role in brain damage.10) We also reported that polyphenols such as luteolin and curcumin had inhibitory effects against the in vivo brain oxidative stress model.11,12) However, it has not been examined whether compounds that activate the Nrf2–ARE pathway have preventive effects in the model. Therefore, in the present study, we examined whether orally administered DMF, which activates the Nrf2–ARE pathway, prevented the oxidative stress induced by SNP in the brain.
Experiments were performed using 6- to 8-week-old male ICR mice weighing 25–30 g; these mice were purchased from Nihon SLC (Shizuoka, Japan). Sodium nitroprusside, 2,3,5-triphenyltetrazolium chloride (TTC), and dimethyl sulfoxide were purchased from Nacalai Tesque (Kyoto, Japan). L-Buthionine-[S,R]-sulfoximine (BSO) were obtained from Sigma (St. Louis, MO, U.S.A.). Zinc–protoporphyrin IX (Zn–PP IX) was obtained from Calbiochem (San Diego, CA, U.S.A.).
Animals and Oral AdministrationThe experiments were performed in accordance with the Ethical Guidelines of the Kyoto University Animal Experimentation Committee and under the guidance of the Japanese Pharmacological Society. This study was approved by the Kyoto University Animal Experimentation Committee. Mice were orally administered DMF (60–200 mg/kg in 0.8% methyl cellulose) using a gavage. On the day after the administration, SNP (10 nmol in 0.9% saline) was injected into the striatum of the mice, and 24 h later, behavioral tests and TTC staining were performed.
Rotarod TestRotarod test is used to assess motor coordination and balance in mice. In the present study, the rotational speed tested in the steady-state mode was 20 rpm for 180 s. The time for which each mouse was able to walk on the rod before falling was determined.
Locomotor Activity TestLocomotor activity test is used to assess spontaneous activity in mice. The locomotor activity was individually tested in an open field using boxes equipped with infrared beams. Mice were placed into a box and their movements were measured for 5 min. The number of interruptions of photo beams for 5 min per mouse was registered as the number of transitions (horizontal activity). In the same time, the number of rearings (vertical activity) was counted.
Surgery and Intrastriatal MicroinjectionMice were anesthetized with sodium pentobarbital (60 mg/kg, intraperitoneally (i.p.)) and placed in a stereotaxic frame. An incision was made along the midline of the skull. Coordinates for injections were as follows: AP, +0.4 mm; ML, +2 mm; and DV, −3.5 mm. After injection, the needle was held in place for additional 5 min to prevent backflow and allow diffusion of the drugs.
Histopathological ExaminationBrains were immediately removed after intracardial infusion of phosphate-buffered saline (PBS) and coronally cut; the brain slices were immediately incubated in 2% TTC solution for 30 min at 37°C. The unstained areas in slices were quantified using the Image J 1.42 program, and the volume of damage was calculated by summing up the damaged areas in all slices.
Cell CulturePrimary cell cultures were prepared from the striatum of fetal Wistar rats on gestational days 17–19 in accordance with a previously described method.13) In brief, the striatum of the fetal rats was removed, minced, and filtered through a stainless steel mesh to obtain a single-cell suspension. Striatal cells were then cultured in culture plates coated with 0.1% polyethylenimine. Cultures were maintained in Eagle’s minimal essential medium (MEM) supplemented with 10% heat-inactivated fetal bovine serum at 37°C in a humidified atmosphere of 95% O2 and 5% CO2. Cells were treated with DMF for 24 h prior to the addition of H2O2.
Determination of Cell ViabilityCell viability was assessed by measuring the amount of lactate dehydrogenase (LDH) released into the culture medium using the cytotoxicity detection LDH kit, in accordance with a previously described method.13) In brief, culture supernatant was added to the LDH substrate mixture in a 96-well plate and incubated. Subsequently, the reaction was stopped and the absorbance was read at 570 nm using a microplate reader (Bio-Rad, Hercules, CA, U.S.A.). Cell viability was analyzed relative to the total amount of LDH released by exposure to 1 mM H2O2 for 24 h.
Statistical AnalysesData are expressed as the mean±standard error of the mean (S.E.M.). One-way ANOVA was used, followed by Tukey’s or Dunnett’s post test to determine statistical significance. Results were considered statistically significant at p<0.05. All statistical analyses were conducted using GraphPad InStat (GraphPad Software Inc., San Diego, CA, U.S.A.).
To evaluate the effect of the oral administration of DMF on SNP-induced motor dysfunction, 24 h after the administration of DMF, SNP (10 nmol) was injected into the striatum of mice. As shown in Fig. 1A, the oral administration of DMF (60–200 mg/kg) dose dependently prevented the SNP-induced impairment in mice performance, as determined using the rotarod test. In the locomotor test, DMF did not affect horizontal activities (transitions) but significantly increased vertical activities (rearings) (Figs. 1B, C).
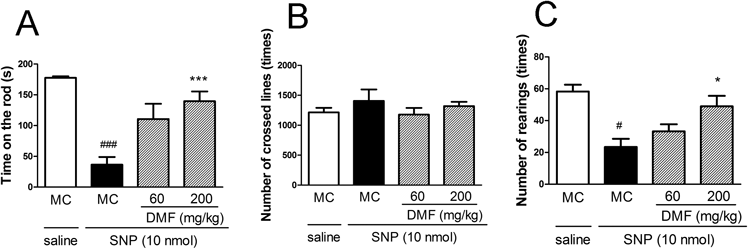
DMF or methyl cellulose (MC) was orally administered 24 h before the microinjection of SNP (10 nmol). Rotarod test (A), open field test (B), and rearing test (C) were performed 24 h after the microinjection into the striatum. n=9–11, # p<0.05, ### p<0.001, compared with saline. * p<0.05, *** p<0.001, compared with SNP.
Next, we investigated the effect of orally administered DMF on brain damage induced by SNP. As shown in Fig. 2, the quantitative analysis of TTC staining showed that the oral administration of DMF (60–200 mg/kg) dose dependently protected against brain damage induced by SNP.

(A–C) Representative images of TTC. (A) Methyl cellulose (MC)+saline, (B) MC+SNP, and (C) DMF (200 mg/kg)+SNP. DMF was orally administered 24 h before the microinjection of SNP (10 nmol) into the striatum. TTC staining was performed 24 h after the microinjection. The brains were sliced into coronal sections for TTC staining. (D) Quantitative analysis of damaged volume based on TTC staining. n=9–11, ## p<0.01, compared with saline. * p<0.05, compared with SNP.
To determine the nontoxic concentrations of DMF, cells were treated with various concentrations of it for 48 h. DMF did not show toxicity at 10 µM (Fig. 3A). The effect of DMF against H2O2-induced cytotoxicity was examined in rat striatal primary cells. As shown in Fig. 3B, treatment with DMF (10 µM) significantly attenuated H2O2-induced cytotoxicity. These results suggest that DMF has the potential to protect striatal cells against H2O2-induced oxidative stress.

(A) Cells were treated with DMF (0.1–10 µM) for 48 h. (B) Cells were treated with DMF (0.1–10 µM) for 24 h and then exposed to H2O2 (30 µM) for 24 h. ### p<0.001, compared with vehicle. * p<0.05, compared with H2O2.
Our previous study demonstrated that both heme oxygenase-1 (HO-1) and γ-glutamylcysteine synthetase (γ-GCS) are up-regulated via the activation of the Nrf2–ARE pathway in striatal cells.7) Thus, we next examined whether HO-1 and γ-GCS were involved in the cytoprotective effect of DMF. First, we investigated the involvement of HO-1 in the protective effect of DMF. The co-administration of 3 µM Zn–PP IX, a competitive HO-1 inhibitor, significantly suppressed the protective effect of DMF (Fig. 4A). We next examined the effect of BSO, an irreversible inhibitor of γ-GCS, on cytoprotection by DMF. BSO did not influence the protective effect of DMF against H2O2-induced cytotoxicity (Fig. 4B).

(A) Cells were treated with DMF (10 µM) and ZnPP (3 µM) for 24 h and then H2O2 (30 µM) was added for 24 h. (B) Cells were treated with DMF and BSO (0.1 µM) for 24 h and then H2O2 (30 µM) was added for 24 h. n.s.: not significant. ### p<0.001, compared with vehicle. * p<0.05, ** p<0.01, compared with H2O2. +p<0.05.
In the present study, we demonstrated that orally administered DMF inhibited brain damage and motor dysfunction induced by the microinjection of SNP. A previous study has reported that orally administered DMF improves the motor dysfunction caused by multiple sclerosis.8) However, the mechanisms underlying this effect of DMF have not been comprehensively investigated. In that study, DMF was chronically administered.8) In this study, we examined the effect of DMF on acute oxidative stress induced by SNP (in vivo study) and H2O2 (in vitro study). Our in vivo study showed that pretreatment with DMF for only 1 d could prevent brain damage. We previously reported that typical antioxidants such as luteolin and curcumin had protective effects in the same animal model.11,12) However, whether the reagents that activate the Nrf2–ARE pathway could prevent brain damage was not determined. This study indicated that agents activating the Nrf2–ARE pathway, including DMF, could protect the brain against the oxidative stress induced by SNP.
Next, we examined the effect of DMF on oxidative stress in primary striatal cells to elucidate the protective effect of this drug. First, we investigated the involvement of HO-1 in the protective effect of DMF. Zn–PP IX inhibited DMF-induced cytoprotection in striatal cells. On the other hand, BSO did not affect the DMF-induced cytoprotection. These results suggested that the increased activity of HO-1 plays a crucial role in the cytoprotective effect of DMF.
DMF showed the protective effect against oxidative stress both in vivo and in vitro study. However, DMF had only limited protection against H2O2-induced cytotoxicity of striatal culture, compared to in vivo effects. The discrepancy between the in vivo and in vitro effect of DMF was considered that different reactive oxygen species (ROS) would contribute to damage of in vivo and in vitro. Moreover, our data suggest that DMF increased HO-1 activity. HO-1 catalyzes degradation of heme to yield biliverdin, free iron and carbon monoxide. Biliverdin is subsequently converted to bilirubin through the actions of biliverdin reductase.14) Bilirubin serves as a potent radical scavenger and protects against oxidative stress.15,16) Bilirubin did not prevent H2O2-induced cytotoxicity using this cultured striatal cells.7) Thus, increased activity of HO-1 may not show prominent protective effect in vitro study.
In conclusion, our findings suggest that orally administered DMF could prevent oxidative stress in the brain and that the increased activity of HO-1 plays an important role in the cytoprotective mechanism of DMF. These results suggest that the activation of the Nrf2–ARE pathway in brain could prevent the telencephalic damage caused by oxidative stress.
This study was supported in part by JSPS KAKENHI Grant No. 24390139 and by a Grant from the Smoking Research Foundation, Japan.
The authors declare no conflict of interest.