2016 Volume 39 Issue 6 Pages 1077-1080
2016 Volume 39 Issue 6 Pages 1077-1080
Zerumbone derivatives 1–4 are 11-membered cyclic compounds synthesised from a sesquiterpene zerumbone obtained from the rhizomes of Zingiber zerumbet SMITH (Zingiberaceae). In this study, we investigated the locomotor-reducing effects of hexahydrozerumbone derivatives 1–3 and zerumbol 4, and those of β-caryophyllene 5 and caryophyllene oxide 6, which are present in Z. zerumbet essential oil. The absence of the double bond at C6 weakened the locomotor-reducing effects. β-Caryophyllene 5 and caryophyllene oxide 6 showed locomotor-reducing effects in mice at 4.5×10−3 mg/cage. Moreover, locomotor activity increased significantly at 0.45 mg/cage of caryophyllene oxide 6.
Zerumbone obtained from Zingiber zerumbet SMITH (Zingiberaceae) and its derivatives are highly volatile compounds with distinct aromas.1,2) They show locomotor-reducing effects when inhaled, and their structure–activity relationships have been investigated.3) The synthesis of hexahydrozerumbone derivatives 1–3, which also have distinct aromas,1) was achieved by hydrogenation of the C6 double bonds of tetrahydrozerumbone (THZ) derivatives.
Takemoto et al.4) reported locomotor-reducing effects when bicyclic and tricyclic terpenoids are inhaled by mice. Thus, the compounds β-caryophyllene 5 and caryophyllene oxide 65) contained in the rhizomes, leaves and flowers of Z. zerumbet, whose structures are similar to zerumbone’s structure, could also show locomotor-reducing effects. In this study, the locomotor-reducing effects of the new zerumbone derivatives 1–3 and caryophyllene derivatives 5 and 6 were investigated. Furthermore, the effects of 8-hydroxyl-α-humulene (zerumbol) 4, an expected intermediate in the biosynthesis of zerumbone6) from α-humulene, were also investigated.
Compounds 1–6 (Fig. 1) were prepared as follows. Hexahydrozerumbone (HHZ) derivatives and zerumbol 1–4 were derived from the starting material zerumbone, which was obtained from the rhizomes of Z. zerumbet SMITH. First, zerumbone was hydrogenated under an H2 atmosphere using Pd/C in hexane to give 2,3,6,7,10,11-hexahydrozerumbone 1. Compound 1 was reduced with NaBH4 in ethanol to give hexahydrozerumbol 2. Compound 2 was acetylated with acetic anhydride and pyridine to give hexahydrozerumbyl acetate 3. Racemic zerumbol 4 was obtained by reduction of zerumbone with LiAlH4 in dry tetrahydrofuran. Compounds 1–4 were each refined to more than 99.9% purity, as evaluated by 1H-NMR.7) β-Caryophyllene 5 was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan), and caryophyllene oxide 6 was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). The odourless solvent triethyl citrate used to dissolve and dilute the fragrant compounds for inhalation was purchased from Merck KGaA (Darmstadt, Germany). The reagents used in this study were of the highest grade available.

1, hexahydrozerumbone; 2, hexahydrozerumbol; 3, hexahydrozerumbyl acetate; 4, zerumbol, 5, β-caryophyllene; 6, caryophyllene oxide.
The animal studies were approved by the Animal Research Committee of Kyoto University, Kyoto, Japan (authorisation numbers: 2014–17). Four-week-old male ddY mice (about 25 g each) were purchased from Japan SLC (Shizuoka, Japan). The mice were housed in colony cages (4 mice per cage) at an ambient temperature of 25°C under a 12 h light–dark cycle. They were fed standard pellet chow and water ad libitum. All behavioural observations were conducted between 09 : 00 and 17 : 00.
MethodsEach compound was dissolved in triethyl citrate (400 µL) and dropped onto four filter paper disks, which were then attached to the four top corners of the glass cage (61.2 L). The vapour from the solution was allowed to fill the cage by natural diffusion for 60 min. A mouse was then placed in the centre of the cage and monitored with a video camera for 60 min. The total spontaneous locomotor activity (TLA) is represented by the area under the curve, which was calculated from a graph with time (min) on the x-axis, and the number of times per 5 min the mouse crossed lines drawn on the bottom of the cage at 10 cm intervals on the y-axis.4) Most of the effective compounds showed a two-phase effect and effects at lower doses were considered true activity because the mice displayed excited activities, such as jumping and rearing, at higher doses.8)
Statistical AnalysisResults were expressed as the mean±standard error of the mean (S.E.M.). Statistical analyses were performed by Dunnett’s test using GraphPad Instat (GraphPad Software, San Diego, CA, U.S.A.).8) A probability level of p<0.05 was considered statistically significant.
Inhalation of HHZ 1 significantly reduced TLA at a dose of 4.5×10−3 mg/cage (Fig. 2a). Inhalation of hexahydrozerumbol 2 significantly reduced TLA within the dose range 4.5×10−4 to 4.5×10−2 mg/cage, while the strongest reduction of TLA was observed at 4.5×10−3 mg/cage (Fig. 2b). In the case of hexahydrozerumbyl acetate 3, a significant reduction in TLA was observed at only a dose of 0.45 mg/cage. No excited activities like rearing and jumping were noted, so this was considered a true effect (Fig. 2c). Exposure to zerumbol 4 did not reduce TLA significantly at any dose (Fig. 2d).
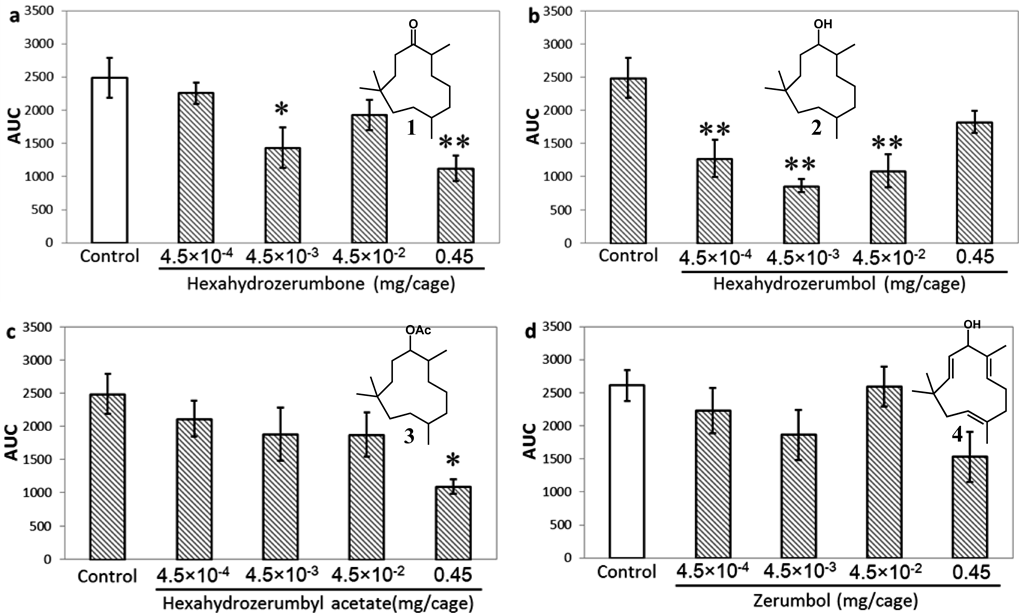
Data are expressed as the mean±S.E.M. for 5 mice. Statistical analysis was performed using one-way ANOVA followed by Dunnett’s test. * p<0.05 and ** p<0.01 vs. the control group. AUC, area under the curve.
Both β-caryophyllene 5 and caryophyllene oxide 6 produced a significant reduction in TLA at doses of 4.5×10−3 mg/cage. β-Caryophyllene also significantly reduced TLA at a dose of 0.45 mg/cage. However, this result was not regarded as a true locomotor-reducing effect, because the mice showed excited activities, namely, continuous rearing. In the case of caryophyllene oxide, a significant increase in TLA, including jumping and rearing activities, were observed at a dose of 0.45 mg/cage (Fig. 3).

Data are expressed as the mean±S.E.M. for 5 mice. Statistical analysis was performed using one-way ANOVA followed by Dunnett’s test. * p<0.05 and ** p<0.01 vs. the control group. AUC, area under the curve.
The THZ derivatives reduced TLA at 10-fold lower doses than HHZ derivatives as shown by a comparison between the HHZ derivatives and THZ derivatives in our previous study. This would suggest that the double bond at C6–C7 contributes to the locomotor-reducing effects of THZ derivatives at lower doses.3) Our previous study demonstrated that ring structures and geometrical isomers of diastereomeric compounds influence the activities of the compounds.3) These prior results along with the results of this study indicate that the double bond at C6–C7 is essential, and the ring structure strongly influences the activities of the compounds.
Influence of Replacing the Ketone with a Hydroxyl Group on Locomotor ActivityZerumbol showed a tendency to reduce TLA at doses of 4.5×10−3 and 0.45 mg/cage, but these results were not significant. Zerumbone and α-humulene showed similar effects on the reduction of TLA in our previous study,3) yet the reduction of TLA via zerumbol differed from the effects of the compounds in our previous study. The strongest locomotor-reducing effect of zerumbol occurred at a higher dose than that of zerumbone. This effect might have been caused by the hydrogen bonds between two hydrogen groups of zerumbol. In fact, the melting point of zerumbol is higher than that of zerumbone,9,10) and when the ketone group was replaced with a hydroxyl it weakened the locomotor-reducing effects of THZ.3) These results suggest that higher boiling points are linked to reduced vaporisation, which might contribute to the weakened locomotor-reducing effects of zerumbol.
Influence of an Epoxy Group on Locomotor ActivitiesA comparison of the locomotor-reducing effects of β-caryophyllene with those of caryophyllene oxide showed that both compounds significantly reduced locomotor-activities in mice at a dose of 4.5×10−3 mg/cage, and the locomotor-reducing effects of caryophyllene oxide were weaker than those of β-caryophyllene. This suggests that the epoxy group might have weakened the locomotor-reducing effects. β-Caryophyllene reduced locomotor activities at a dose of 0.45 mg/cage, while the activities of caryophyllene oxide significantly increased the locomotor effects at the same dose. Previous studies indicate that excited activities like jumping and rearing are observed in mice following inhalation of fragrant compounds at high doses.3,4,8,11,12) Furthermore, locomotor activities can increase when mice inhale essential oils and compounds without locomotor-reducing effects.13) Tankam and Ito12) revealed that Ocimum gratissimum essential oil, which shows locomotor-reducing effects at doses of 4.0×10−8 to 4.0×10−6 mg/cage, increased the locomotor activities of mice at quite low doses, although the result was not significant. This is the first report to show that compounds with locomotor-reducing effects can significantly increase locomotor activities in mice at different doses. Previous reports and our results suggest some compounds induce both locomotor-reducing and locomotor-enhancing effects. Comparison of the structures of β-caryophyllene and caryophyllene oxide and their locomotor activities after inhalation at a dose of 0.45 mg/cage suggests that the epoxy group contributes to the locomotor-enhancing effects of caryophyllene oxide.
Oxygen-containing groups and the absence of double bonds at C2–C3 and C10–C11 are important for the locomotor-reducing effects of zerumbone derivatives. Furthermore, acetylation of oxygen-containing groups weakens these effects, and trans isomers show stronger effects than cis isomers.3) According to the results of our previous study, isomers of hexahydrozerumbone derivatives 2 and 3 with trans configuration in C1 and C2 are supposed to reduce locomotor activities in mice stronger than other types of isomers. And racemic, (R)- and (S)-tetrahydrozerumbones were reported that they have showed similar locomotor-reducing activities.3) This may indicate that hexahydrozerumbone 1 with any enantiometric configuration in C2 will show locomotor-reducing activities. This study revealed that the double bond at C6–C7 is important for strong locomotor-reducing effects. Our results suggest that the conformations of the compounds strongly affect their locomotor-reducing effects; therefore, an investigation into the interactions between zerumbone derivatives and receptors via X-ray crystallography would provide valuable information for the synthesis of compounds with improved locomotor-reducing effects. Caryophyllene derivatives were shown to reduce locomotor activity, which indicates that the locomotor-reducing effects of essential oils in Perilla frutescens14) and Mentha×piperita,15) which contain caryophyllene derivatives, might be due to these compounds.16,17) In addition, from these perspectives, it may be natural to consider the enhancement of locomotor activity by high concentrations of caryophyllene oxide as an adverse event.3,4,8,11,12) At the same time, however, these results suggest the existence of compounds that are capable of significantly enhancing locomotor activity.
We would like to thank Mr. N. Ukeda for his contribution to this study.
The authors declare no conflict of interest.