2016 年 39 巻 7 号 p. 1206-1210
2016 年 39 巻 7 号 p. 1206-1210
In vitro permeation studies of mannitol were conducted across excised hairless mouse skin to determine and compare the enhancing effect of electroporation (EP) or sonophoresis (SP) combined with iontophoresis (IP) on the electroosmotic flow, and to analyze the enhancement mechanism of these combined methods. Mannitol flux was utilized as an index for the electroosmotic flow due to its low molecular weight and no electrorepulsion effect. The combination of SP and IP (SP/IP) resulted in an apparent increase of electroosmotic flow (no effect was sometimes observed by SP/IP), while that of EP and IP (EP/IP) had no synergistic enhancing effect on the electroosmosis. Next, the combined effect of tape-stripping (TS) and IP (TS/IP) was examined in a similar manner to clarify the difference between the SP/IP and EP/IP effects on electroosmosis. When the TS number increased from 0 to 3, the electroosmotic flow increased with the TS number. However, no further increase was observed when the TS number became more than 3, and the flow started to decrease when the TS number became 5. The electric charge of the skin surface was then measured after SP or TS application. When SP was applied, the skin surface charge became much more negative and the electroosmotic flow by SP/IP was markedly increased. Thus, an increase in the electroosmotic flow across the skin during IP application can be obtained not by EP and TS, but by SP. The combined use of SP and IP is a promising means for the enhanced skin delivery of non-electrolyte drugs.
Recently, advances in development and practical application of physical transdermal drug delivery techniques have been achieved in iontophoresis (IP), sonophoresis (SP) and electroporation (EP). Although conventional transdermal delivery is based on passive diffusion using the concentration gradient of drugs through the skin barrier after topical application, these physical means are characterized by active drug administration based on physical perforation of the skin barrier using external energy sources, and can be used to deliver peptides, proteins and other macromolecular drugs as well as low-molecular-weight organic drugs.
Among these techniques, IP1) and EP2) are based on electrical energy. IP, involving the application of a weak current for a relatively long period, is a means of using electrical repulsive force (electrorepulsion) and electroosmotic flow (electroosmosis) as driving forces.3) EP, involving the application of a high voltage (100 to 1000 V) for a very short period (100 µs to 100 ms), is used to form new aqueous pathways in the lamellar structure of the stratum corneum.2,4) The EP effect depends on the electric field applied to the skin.5)
SP6) is a skin-penetration-enhancing technique involving the application of a low frequency to the skin surface through media such as aqueous solution.
Recently, several combined trials of EP/IP have been reported using insulin,7) 5-fluorouracil8) and calcitonin,9) and those of SP/IP using heparin.10) According to these reports, EP or SP was applied as a pretreatment to create new aqueous pathways on the skin barrier, prior to IP application. It is important to utilize electrorepulsion and electroosmosis effectively in IP, as these are two major driving forces for IP. Although electrorepulsion is important for the skin permeation of ionic drugs, electroosmosis is also very important, especially where the skin permeability for uncharged and/or high-molecular-weight compounds is concerned. The electroosmotic flow, a convective solvent flow, is obtained by electroosmosis. The skin permeation-enhancing mechanism by electroosmosis is based on the net negative charge at the skin surface when a physiological pH (7.4) is applied to the skin. Thus, the direction of the solvent flow depends significantly on the isoelectric point (pI) of the skin membrane, which in turn depends on the pH of applied aqueous solution.11) In addition, electrosmosis flow was significantly affected by additives in the formulation or applied physical means.12,13) We have already examined the effect of EP pretreatment on electroosmosis and reported that the electroosmotic flow decreased with an increase in the applied strength of EP.14)
On the other hand, the effect and mechanism of SP on electroosmosis are still unknown, so they must be elucidated during IP application in detail. Discussing the mechanism of enhancement for the combination of SP/IP is very important to examine the relationship between the effects of SP on the skin barrier and electroosmosis. In the present study, therefore, electroosmosis across skin was compared among IP alone, EP/IP and SP/IP combinations in order to assess the influence of EP/IP and SP/IP on the electroosmotic flow, where mannitol flux was used as an index of electroosmosis across the skin. Moreover, zeta-potential of the skin surface treated by SP or tape-stripping (TS) was measured to discuss the relationship between electroosmosis and skin surface charge.
D-Mannitol and N-(2-hydroxyethyl) piperazine-N′-2-ethanesulfonic acid (HEPES) buffer were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). D-[1-14C]Mannitol (specific activity: 56.0 mCi/mmol) was purchased from Amersham Pharmacia Biotech (Buckinghamshire, U.K.). Ag and Ag/AgCl electrodes (0.04 mm thick) were prepared with silver wires (Murata Yohaku, Tokyo, Japan) and used in the IP application. Frog-type electrodes were used in EP application.15) Nichiban’s cellophane tape (Cellophane Tape™; Nichiban, Tokyo, Japan) was used in the tape-stripping of stratum corneum from skin. Female hairless mice (HR-1 strain) of 7–8 weeks old obtained from SLC (Hamamatsu, Shizuoka, Japan) were used in all animal experiments. All animal studies were conducted according to the recommendations of the Institutional Board for Animal Studies, Josai University (Sakado, Saitama, Japan).
In Vitro ExperimentFreshly removed hairless mouse skin was mounted on a Franz cell (skin area: 1.77 cm2, receiver volume: 16 mL) with its stratum corneum side up. The skin permeation study was conducted using a skin sample wiped with ethanol-impregnated absorbent cotton to eliminate the influence of strains on the skin surface. The donor and receiver chambers were filled with 25 mM HEPES with 133 mM NaCl, the pH of which had been adjusted to 7.4 in advance (HEPES pH 7.4).
Physical Pretreatments of EP, Low-Frequency SP and TS of Stratum CorneumA square pulse generator (Electro Square Porator T820; BTX, San Diego, CA, U.S.A.) was used for EP pretreatment. A voltage of 150 V was applied 10 times for a very short period (1.0 ms) at a pulse interval of 1.0 s using frog-type electrodes in this EP application. A sonicator with a low frequency of 20 kHz (VCX400; horn area: 1.33 cm2; Sonics & Materials Inc., Newtown, CT, U.S.A.) was also used for the SP pretreatment to skin instead of EP pretreatment. The sonicator horn was set in donor solution at approximately 5 mm above the skin, and ultrasound was applied at 1.1 W/cm2 for 1.0 min under the following conditions: 5 s of ON/OFF pulse mode. Adhesive tape (Cellotape™) was put on the excised hairless mouse skin, and peeled off a few layers of stratum corneum in the case of TS pretreatment. This operation was repeated up to five times. After the EP, SP or TS pretreatment, the donor solution was replaced with 1 mM mannitol solution (1 µCi/mL 14C-mannitol-HEPES, pH 7.4) to start the permeation experiment. Immediately after the pretreatment, 4-h passive diffusion was determined.
In the IP treatment, 0.4 mA/cm2 direct current was applied for 4 h with ADIS-HP (Advance Iontophoresis System, Hisamitsu Pharmaceutical Co., Inc., Saga, Japan) after the 4-h permeation experiment. The Ag and Ag/AgCl wire electrodes were used as an anode and a cathode, respectively. These electrodes were set according to our previous paper.14)
The skin permeation experiment also proceeded for 4 h more after finishing the IP application. Two hundred microliters of the receiver solution was pipetted at constant intervals, and the same amount of fresh buffer was added to prepare samples. The receiver solution samples were measured for 14C-mannitol concentration with a liquid scintillation counter (LSC-5100; Aloka, Tokyo, Japan) to determine the flux across the skin. Each permeation experiment was repeated at least three times.
Surface Charge of Hairless Mouse SkinZeta-potential of the hairless mouse skin surface was determined using an electrophoretic light scattering spectrophotometer (ELS-8000; Otsuka Electronics Co., Tokyo, Japan) equipped with a plated sample cell. Skin samples were cut squarely 1 cm×2.5 cm size. The measurement was carried out in 0.01 M KCL solution with polystyrene latex monitor particles. Each data was determined from the mean value of the measured results that automatically repeated plurality of times under the same condition.
Figure 1 shows the time course of mannitol flux through the excised hairless mouse skin in groups treated with IP alone and EP/IP and SP/IP combinations. Skin permeation experiments were performed over 12 h: Passive diffusion of mannitol immediately after EP or SP application was firstly determined for 4 h, active permeation of mannitol with IP was then determined over another 4 h, and finally passive diffusion was then determined for a further 4 h. First, the average passive mannitol flux that calculated from the cumulative amount of permeation through the skin between 0 to 4h application period was compared to evaluate the pretreatment effect with EP and SP, and then the average flux during IP application (4–8 h) with or without pretreatment was evaluated. Compared with the average passive flux of mannitol without EP and SP pretreatment (3.0 ng/cm2/h), approximately 122 times greater flux (366 ng/cm2/h) was observed by the pretreatment of 150 V EP. On the other hand, low-frequency SP pretreatment showed a very wide distribution of skin permeation despite the same conditions being used (1.1 W/cm2). Thus, the SP pretreatment experiment was sufficiently repeated (n=16). Consequently, the individual experimentally obtained passive flux value after SP pretreatment was distributed widely, from about 0.7 to 267 times (2.2 to 800 ng/cm2/h) compared with the mean passive flux of no pretreatment with IP group (3.0 ng/cm2/h). Interestingly, when IP was applied onto the SP treated skin, a very high flux (804 ng/cm2/h) was observed during IP application through SP pretreatment skin compared with the IP flux of EP pretreatment group (588 ng/cm2/h). However, the distribution of individual experimentally obtained flux value varied widely (226 to 2046 ng/cm2/h) as is the case in IP flux with SP pretreatment. In addition, the flux after quitting IP application was recovered to that before IP application.

EP (10 pulses of 150 V, 1 ms) and SP (5 s ON/OFF for 60 s) were applied at the beginning of each study. Symbols: □, no pretreatment with IP; ◇, EP pretreatment at 150 V with IP; ○, SP pretreatment with IP. Each data point represents the mean±S.D. of 3 to 5 experiments, except for SP with IP (n=16).
In the case of no pretreatment, mannitol flux was increased by the IP application, but the flux was recovered to values before IP application when quitting the IP application. Although the groups with EP pretreatment showed the same tendency, the flux during IP application was not so marked, probably due to high flux just with EP pretreatment.
No exact reason was found for the unexpected wide distribution in the fluxes of SP pretreatment groups. However, the mannitol flux for the SP/IPcombination (JSP/IP) was remarkably increased when the passive flux for SP pretreatment (JSP) was high.
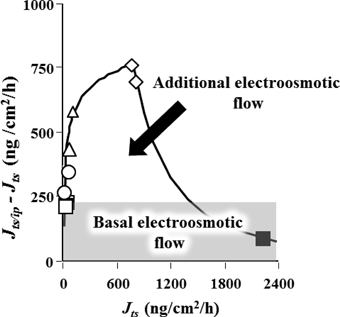
Figure 2 shows the calculated results using the individual flux value before and during IP application. The flux difference, JSP/IP–JSP, must be the sum of “intrinsic electroosmotic flow” and “additional electroosmotic flow,” as shown in the figure. Thus, this additional electroosmotic flow must have been high when the passive diffusion (JSP) was high. In addition, this additional flux showed a convex curve against JSP (Fig. 2).

Subsequently, permeation study of mannitol was conducted using tape-stripping (TS) of skin. The numbers of TS treatments for the skin were set to be 0, 1, 2, 3 and 5. Table 1 summarizes the passive flux (JTS) and the flux (JTS/IP) during IP across the TS treated skin. The passive flux and electroosmotic flux increased with the number of TS treatments. The mannitol flux hardly changed from that of the intact skin (data not shown). With these experimental data, (JTS/IP–JTS) and JTS for TS pretreatment were determined to estimate the relationship between passive flux and electroosmotic flow. Figure 3 shows the relationship between (JTS/IP–JTS) and JTS for 0–5 TS treatments. The value of (JTS/IP–JTS) increased with an increase in JTS (with an increase in the number of TS treatments), but decreased when JTS exceeded 700 ng/cm2/h (when the number of TS treatments was more than 3). These results are consistent with our previous results,15) demonstrating that complete removal of the stratum corneum leads to the disappearance of electroosmotic flow.
| Passive flux (JTS) (ng/cm2/h) | JTS/IP (ng/cm2/h) | |
|---|---|---|
| Control | 2.97±1.0 | 255±11 |
| Stripping-1 | 19.3±5.7 | 285±22 |
| Stripping-2 | 67.9±9.8 | 548±16 |
| Stripping-3 | 718±47 | 1524±55 |
| Stripping-5 | 1486±121 | 1947±162 |
Each data point represents the mean of 2 to 3 experiments. The flux value that obtained from control represents the mannitol flux through the skin without tape-stripping treatment.
Symbols: □, TS-0; ○, TS-1; ◇, TS-2; △, TS-3; ■, TS-5.
In order to consider these results further, the enhancement ratio (ER) of the mannitol flux (4–8 h) during IP application against that after IP application (8–12 h) was calculated. Figure 4 shows the results. ER for the SP/IP combination, for example, is expressed by the following formula: (JSP/IP–JSP)/(JIP–JPassive). The values of ER for the EP/IP and TS/IP combinations were calculated in the same manner. In the SP/IP and TS/IP combinations, ER increased with an increase in passive flux: ER decreased in the EP/IP combination. ER in the SP/IP combination showed the largest value (more than 5 times that in the other groups).

ER was calculated by dividing the electroosmotic flux (J4–8) by the passive flux (J8–12) after IP.
The zeta potential of the skin surface pretreated by TS or SP was measured using an electrophoretic light scattering spectrophotometer. Table 2 shows the results. While the zeta potentials of the control and EP-pretreated skin surfaces were approximately −4.0, that of the TS-treated skin surface ranged roughly between −1.5 and −4.0. Thus, these skin surfaces must be similar to that of the control or rather neutralized skin surface. In contrast, zeta potential in the SP group was approximately −8.0, which is much lower than for EP- and SP-pretreated skin surface.
| ζ-Potential | Average | |
|---|---|---|
| Control | −4.37 | −4.58 |
| −4.79 | ||
| Stripping-1 | −1.63 | −1.53 |
| −1.43 | ||
| Stripping-3 | −1.47 | −2.69 |
| −3.90 | ||
| Sonophoresis | −7.06 | −8.63 |
| −7.52 | ||
| −11.32 |
EP and SP involve the physical creation of transient new aqueous pathways (pores) in the stratum corneum, and thus these mechanisms for enhancing the skin permeation of drugs are very different from those of IP.2,4) The present results suggest that the mannitol flux was enhanced synergistically by SP and IP due to higher convective solvent flow produced by electroosmosis during the anodal IP. In addition to our previous results that investigated for the penetration enhancement effect by EP/IP,14) the present study suggests that the EP pretreatment attenuated the electroosmotic flow across the skin from the anode to the cathode. We also investigated the pretreatment effect of microneedle on the iontophoretic transport of D2O and demonstrated that little change was observed in the convective solvent flow during IP application by microneedle pretreatment.16)
These experiments and reports suggest that it is very important to understand whether or not the “skin permselectivity” is affected by each physical penetration enhancement method. In contrast to EP and microneedle, the mechanism for the enhancement of SP includes structural disruption of the stratum corneum by vibration and explosion of cavitation bubbles and acoustic jet streaming under SP application.17) Since SP has a strong cleaning effect on the skin surface, it exerts substantial effects of peeling off the surface of stratum corneum consisting of piles of cells like bricks. Indeed, in the present study, additional electroosmotic flow was observed when the skin was treated by a few TS sessions. However, this additional electroosmotic flow was not as high as that in the SP/IP combination. Thus, the skin surface condition may be closely related to the electroosmotic flow. Several reports have already described the relationship between the skin surface charge and electroosmotic flow.12,18) Nevertheless, the relationship between the SP and skin surface charge has not yet been reported. From the present study, this relationship was firstly regarded as an important factor in discussing the experimental results, even when SP was used as a method of pretreating the skin. Indeed, when the skin surface was treated by SP, the negative charge of the skin surface clearly increased, which suggests that SP treatment must contribute markedly to the increase of electroosmotic flow. The same tendency was expected in the TS-treated skin, although the effect was not high compared with that for SP pretreatment.
SP made the net skin surface charge more negative, so SP enhances the permselective properties of cations for the skin. SP may enhance the skin permeability of positive ions including Na+ and K+ that are accompanied by hydration with water.15) Since synergistic effect of SP/IP was related to passive flux following SP pretreatment, additional electroosmotic flow would be affected not only the charge of skin surface but also the number and the size of new permeation pathways created by SP. The present study is insufficient to demonstrate the mechanism behind the synergistic effect of SP/IP completely. Large variation of the SP effect must be an important problem. When the reason for the large variation of the SP effect is clarified, however, the combination of SP/anodal IP should become the most effective method to increase the skin permeation of a wide variety of neutral (or nonionic) drugs. Since some kind of threshold must exist in the appearance of SP effects, further experiments would be needed to reveal the relationship between SP conditions (strength, duration, solution composition, etc.) and this threshold. Future challenges include determining the variation in the flux by SP pretreatment.
Kenji Sugibayashi and Hiroaki Todo received a research grant from Hisamitsu Pharmaceutical Co., Inc.