2016 Volume 39 Issue 7 Pages 1100-1106
2016 Volume 39 Issue 7 Pages 1100-1106
We previously reported that levels of long-chain fatty acids (FAs) including docosahexaenoic acids (DHA) increase in the hypothalamus of inflammatory pain model mice. However, the precise mechanisms underlying the increment of free fatty acids (FFAs) in the brain during inflammation remains unknown. In this study, we characterized FFAs released by inflammatory stimulation in rat primary cultured astrocytes, and tested the involvement of phospholipase A2 (PLA2) on these mechanisms. Lipopolysaccharide (LPS) stimulation significantly increased the levels of several FAs in the astrocytes. Under these conditions, mRNA expression of cytosolic PLA2 (cPLA2) and calcium-independent PLA2 (iPLA2) in LPS-treated group increased compared with the control group. Furthermore, in the culture media, the levels of DHA and arachidonic acid (ARA) significantly increased by LPS stimuli compared with those of a vehicle-treated control group whereas the levels of saturated FAs (SFAs), namely palmitic acid (PAM) and stearic acid (STA), did not change. In summary, our findings suggest that astrocytes specifically release DHA and ARA by inflammatory conditions. Therefore astrocytes might function as a regulatory factor of DHA and ARA in the brain.
Lipid is one of the necessary nutrients to keep homeostasis. Fatty acids (FAs) are the smallest unit that makes up Lipids. FAs are currently generating considerable attention as a major source of energy in the brain. In particular, a continuous supply of FAs is required for cellular metabolism, differentiation, and development of neuron.1–4) FAs served not only as cellular nutrients and cell membrane components but also as lipid-soluble signal molecules, suggesting that FAs play vital roles in a wide variety of physiological function.5,6) The central nervous system (CNS) is enriched in lipids7–10) and, in particular, brain consist of 20% polyunsaturated FAs (PUFAs) such as docosahexaenoic acid (DHA) and arachidonic acid (ARA). Furthermore, these PUFAs mainly relate the physiological function of the brain.
Astrocytes, one of the glial cells, assume a crucial role to maintain brain and neuronal function via releasing glutamate or neurotrophic factors. In CNS, astrocytes but not neurons are the main source of DHA and ARA. DHA and ARA are released from astroglial membranes under basal and stimulated conditions such as neurotransmitters, bladykinin, glutamate, ATP and thrombin and are supplied to the neurons.11–14) In our previous study, we demonstrated that hypothalamic long chain fatty acid (FA) levels increase in the early phase of complete Freund’s adjuvant (CFA)-induced inflammatory pain.15) The DHA level, in particular, is significantly increased compared with the control group. These increases correlated with an increase in glial fibrillary acidic protein (GFAP) expression in the hypothalamus of CFA-treated mice and were inhibited by the suppression of astrocyte proliferation induced by flavopiridol, a cyclin-dependent kinase inhibitor.16) However, it is unclear whether astrocytes really released DHA or other free fatty acids (FFAs) during inflammation.
In this study, we characterized FFAs that released by inflammatory stimulation in a rat primary cultured astrocytes using liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS), and tested the relationship phospholipase A2 (PLA2) and FFAs release on these mechanisms.
The present study was conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals adopted by the Japanese Pharmacological Society. All experiments were approved by the ethics committee for animal experimentation of Kobe Gakuin University (approval number A15-11; Kobe, Japan).
AnimalsFemale Wistar rats (pregnant by 16 or 17 d) were obtained from Japan SLC, Inc. (Hamamatsu, Japan). Rats were housed in cages at 23–24°C with a 12-h light–dark cycle (lights phase, 08 : 00–20:00). Food and water were available ad libitum.
Primary Astrocyte PreparationAstrocytes were prepared from the cerebra of 0- to 2-d-old Wistar rats, as previously described.17) The isolated cells were seeded at 1×104 cells/cm2 in 75 cm2 culture flasks (Nunc, Roskilde, Denmark) and grown in minimal essential medium (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) supplemented with 10% fetal bovine serum (FBS) (Biosera, Boussens, France). To remove small process-bearing cells (mainly oligodendrocyte progenitors and microglia from the protoplasmic cell layer), the culture flasks were shaken at 250 rpm, 37°C overnight for 10–14 d after seeding. The monolayer cells were trypsinized (GIBCO BRL, Palo Alto, CA, U.S.A.) and seeded in 6-well culture plates (Nunc, Roskilde, Denmark) or on 15-mm glass coverslips (Matsunami Glass Ind., Ltd., Osaka, Japan) in 24-well culture plates (Nunc). At this stage, approximately 95% of the cells showed immunoreactivity for GFAP.
Cell Viability TestEffects of lipopolysaccharide (LPS) from Escherichia coli (Simga-Aldrich Co., St. Louis, MO, U.S.A.) on primary astrocytes were assessed with Cell Count Reagent SF (nacalai tesque, Kyoto, Japan). Primary astrocytes seeded at 1×105 cells/mL into 96-well plates. After incubated 24 h, primary astrocytes cultured with serum-free media for 48 h. Next, LPS was added into each well at various final concentrations and incubated for 24 h. After that, we exchanged with fresh culture media and reacted Cell Count Reagent SF for 1 h. Absorbance was measured at 450 nm and 630 nm using Corona grating microplate reader (SH-1200; Corona Electric Co., Ltd., Ibaraki, Japan).
Treatment of Primary Astrocytes in CultureBefore treatment, astrocytes in 6-well culture plates were incubated in serum-free medium for 48 h. LPS was minimally diluted using serum-free medium before treatment. After incubation in serum-free medium, the cells were treated with 100 or 1000 ng/mL LPS for 24 h.
Sample Preparation for the Determination of FFAs in Primary Cultured Astrocytes and Culture MediumThe FFAs in culture medium were freeze dried using FreeZone Freeze Dry system (LABCONCO FZ-12; Labconco, Kansas, MO, U.S.A.) and dissolve in methanol (Wako Pure Chemical Industries, Ltd., Osaka, Japan). The FFAs in primary astrocytes were extracted in methanol. Extracted solutions were centrifuged at 200×g for 5 min at 4°C and collected supernatant.
Comparative FFAs AnalysesThe sample were centrifuged at 15000×g for 15 min. The supernatant was collected and filtrated in sample vial for LC-MS/MS analysis. HPLC separation was performed with an Agilent 1290 Infinity LC (Agilent Technologies, California, U.S.A.) having a CAPCELL PAK UG120 column: 1.0 mm i.d.×150 mm (Shiseido, Tokyo, Japan). The mobile phases were A, 10 mM ammonium formate (pH 3.5) and B, acetonitrile. The eluting gradient was as follows: the column was equilibrated with 13% A, 13% A for 5 min, 13% A to 5% A in 5 min, 5% A for 5 min, 5% A to 13% A in 5 min, 13% A for 5 min. The flow rate was 0.2 mL/min. We used a QTRAP 4500 (AB SCIEX, Massachusetts, U.S.A.) to carry out quantitation. We operated the mass spectrometer in the negative-ion mode. The FFAs were quantified by selective multireaction monitoring (MRM) with a negative ionization mode. The ion spray voltage was −4500 V, the source temperature was 300°C, the declustering potential ranged −70 from −105 V and collision energy ranged −10 from −22 eV for the fragment ions. The peak of each FFAs was monitored by the product ion obtained from [M−H]-ion (i.e., m/z 255→255 for palmitic acid (PAM), m/z 279→279 for linoleic acid; LNA, m/z 281→281 for oleic acid; OLA, m/z 283→283 for stearic acid; STA, m/z 303→303 for ARA, m/z 327→327 for DHA). Each FFAs concentration was evaluated from each calibration curves by absolute calibration curve method.
Immunofluorescence StudiesPrimary astrocytes cultured on glass coverslips were washed with phosphate-buffered saline (PBS) containing 0.1% Triton X-100 (PBST) three times at 5 min intervals and incubated with blocking buffer (3% BSA in PBST) for 1 h at room temperature. The sections were then incubated with specific antibodies against GFAP (mouse monoclonal anti-GFAP, 1 : 10000; Millipore Corp., Billerica, MA, U.S.A.) overnight at 4°C at which time the antibody was diluted with reaction buffer (1% bovine serum albumin (BSA) in PBS). The next day, sections were washed with PBST and incubated at room temperature for 2 h with secondary antibody conjugated with AlexaFluor 594 (goat polyclonal anti-mouse immunoglobulin G (IgG) 1 : 200, Life Technologies, Inc., Carlsbad, CA, U.S.A.) at which time the secondary antibody was diluted with reaction buffer. After these sections were washed with PBST, we stained nuclear with 4′,6-diamidino-2-phenylindole (DAPI) (Boster Biological Tec., CA, U.S.A.) at room temperature for 10 min. Finally, sections washed with PBST and coverslipped with PermaFluor (Thermo Shandon Inc., Pittsburgh, PA, U.S.A.), and immunoreactivity was detected with a confocal fluorescence microscope (FV1000, Olympus Corporation, Tokyo, Japan). In the immunohistochemical control studies, no staining was detected when the corresponding primary or secondary antibody was omitted.
RNA Preparation and Real-Time PCRAstrocytes in 6-well culture plates were incubated in serum-free medium for 48 h before treatment with LPS. Total RNA was isolated from cultured astrocytes using the RNeasy mini kit (Qiagen, Hilden, Germany) and measured it by NanoDrop Lite Spectrophotometer (Thermo Fisher Scientific Inc., Massachusetts, U.S.A.). First-strand cDNA was synthesized from total RNA (500 ng) using the Primescript RT reagent kit (TaKaRa, Shiga, Japan). The mRNA levels were determined using real time-PCR with SYBR Green fluorescent probes using Light Cycler 96 (Roche, Basel, Switzerland). Expression of β-actin was used as an internal standard, and 20 µM each of primer and 20 ng of cDNA were used. The following primers were used: cPLA2 forward primer 5′-(AAC AGA GCA ACG AGA TGG)-3′ and reverse primer 5′-(AAC AGA GCA ACG AGA TGG)-3′18); iPLA2 forward primer 5′-(ATT GAT AAC AGA ACT CGA GC)-3′ and reverse primer 5′-(GAT GAA TCG GCT TCT GAG TA)-3′19); β-actin forward primer 5′-(GAA CCC TAA GGC CAA CCG TG)-3′ and reverse primer 5′-(TGG CAT AGA GGT CTT TAC GG)-3′,18) To determine each relative gene expression, the ΔΔCt method was employed and analyzed. To normalize target gene, β-actin used as a reference gene.
Statistical AnalysesData are expressed as the mean±standard error of the mean (S.E.M.). The statistical significance of differences were analyzed one-way ANOVA followed by Dunnett’s multiple comparison test. Differences resulting in p values <0.05 were considered statistically significant.
The concentration level of LPS (1–1000 ng/mL) did not affect the cell viability (Fig. 1A). GFAP immunoreactivity increased 24 h after exposure to LPS (Figs. 1B–D). The morphology of the astrocytes also showed hypertrophy of the cell-body upon LPS stimulation compared with the vehicle-treated group (Figs. 1E–G). In contrast, astrocytes treated with vehicle did not show morphological changes.
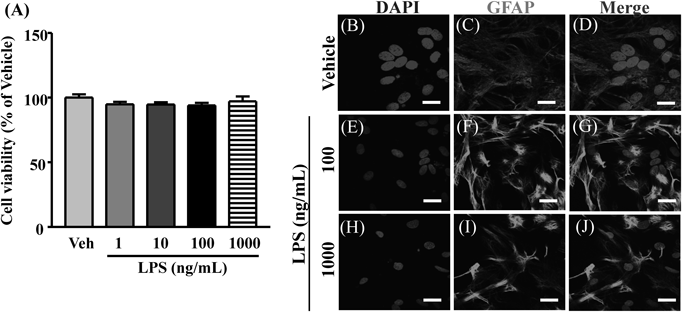
Primary astrocytes treated with LPS at 0, 1, 10, 100, 1000 ng/mL for 24 h. Cell viability assessed using WST-8 assay (A). Data represented mean±S.E.M.; n=4 for each column. Immunofluorescence staining for GFAP (an astrocytes marker) and DAPI (a nuclear marker) in cultured astrocytes from rats after administration of vehicle or LPS (100 or 1000 ng/mL) (B–J). Scale bars: 20 µm (original magnification 100×).
Twenty-four hours after LPS (100 or 1000 ng/mL) stimulation, the levels of PAM, STA, OLA, and LNA dose-dependently increased compared with the vehicle-treated group (Figs. 2B–E). Furthermore, intracellular DHA and ARA levels significantly increased in a dose-dependent manner (Figs. 2F, G).
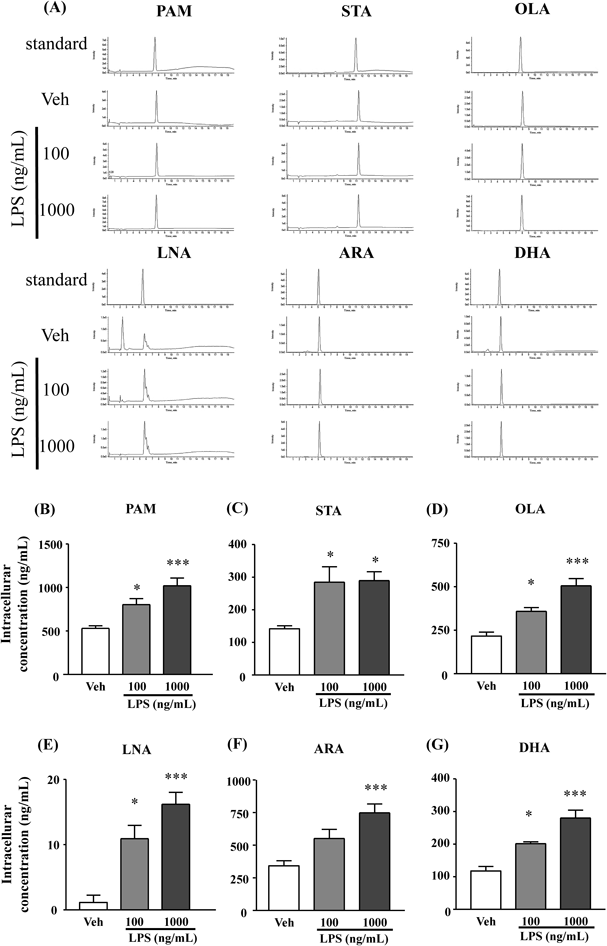
After stimulation with LPS for 24 h, the levels of several FFAs in astrocytes were measured using LC-MS/MS. Represented chromatogram data are shown (A). Quantification of PAM (B), STA (C), OLA (D), LNA (E), ARA (F), and DHA (G). Data represent the mean±S.E.M.; n=3 for each column. * p<0.05, *** p<0.001 compared with vehicle (Dunnett’s test).
After exposure of astrocytes to LPS (100 and 1000 ng/mL) for 24 h, the expression of cPLA2 mRNA significantly and dose-dependently increased compared to the vehicle-treated group (Fig. 3A). Furthermore, iPLA2 mRNA significantly increased after stimulation with 1000 ng/mL LPS but not after stimulation with 100 ng/mL LPS compared with the vehicle-treated group (Fig. 3B).
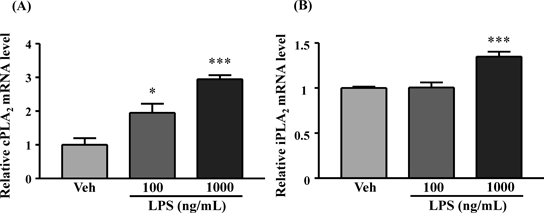
Cultured astrocytes were incubated with 100 or 1000 ng/mL LPS for 24 h. Each sample was measured using real-time PCR. Data represent the mean±S.E.M.; n=3 for each column. * p<0.05, *** p<0.001 compared with vehicle (Dunnett’s test).
DHA, ARA, PAM, STA and OLA were detected in the culture media 24 h after stimulation with 100 or 1000 ng/mL LPS. Levels of the SFAs, PAM and STA as well as levels of the monounsaturated FA OLA did not change upon LPS stimulation (Figs. 4B–E). In contrast, levels of DHA and ARA considerably increased after LPS (1000 ng/mL) stimulation, compared with the vehicle control (Figs. 4F, G).
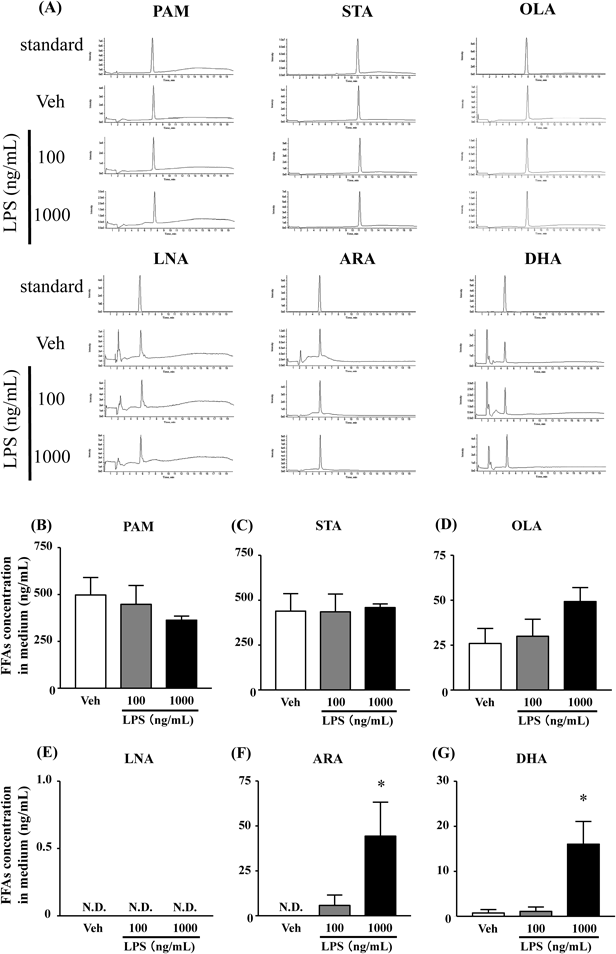
FFAs in culture media were analyzed using LC-MS/MS. Represent chromatogram data are shown (A). Quantification of PAM (B), STA (C), OLA (D), LNA (E), ARA (F), and DHA (G). Data represent the mean±S.E.M.; n=4 for each column. * p<0.05 compared with vehicle (Dunnett’s test).
PUFAs are abundant in the phospholipid bilayer of brain and that they play important roles in brain function and maintenance of its structure.20–22) As previously reported, essential n-3 PUFAs, especially DHA, have beneficial actions in many inflammatory diseases.23–26) For example, in inflamed tissues at peripheral sites, DHA is released from immune cells such as neutrophils during the acute phase of the inflammatory process.27) Most important thing in this study is that LPS stimulation specifically facilitated the release of DHA and ARA from astrocytes although SFAs did not change.
However, in this study, the concentration of DHA and ARA released from astrocyte is nM order, and it is much lower than that of intracellular concentration. Another researchers suggest that DHA shows the beneficial effects in the level of µM in vivo.27) These differences might be due to increment of oxidative metabolites of DHA and ARA in culture. Because, it is well known that PUFAs are readily oxidized in the presence of oxygen. Another possibility is that n-3 FAs metabolites such as eicosapentaenoic acid (EPA)-derived (E-series) or DHA-derived (D-series) resolvins,28,29) which is known as a lipoxygenase and cytochrome P450 metabolites increased for 24 h treatment, whereas the level of DHA is decreased. And also, ARA is easily metabolite via cyclooxygenase or lipoxygenase, and result in induction of the production of pro-inflammatory factors such as prostaglandin E2 and leukotriene,30) and ARA-derived eicosanoids lipoxin which known modulate inflammation to resolve the pro-inflammatory process.29,31) However, it is unclear whether the concentration of ARA and DHA released from astrocytes has beneficial effect such as an anti-inflammation effect or not. Therefore, our results indicate that astrocytes might be involved in control of brain FFAs composition respond to LPS stimulation. Based on our previous reports,32,33) it is thought that these FFAs released from astrocytes might maintain a neuronal function as a ligand of GPR40/FFAR1, one of a FFAs receptors.34)
Next, we examined how FFAs, especially DHA and ARA, increase in astrocytes after LPS stimulation. It is widely accepted that the main site of storage for DHA and ARA is the sn-2 position of membrane phospholipids35) and that enzymes responsible for their release are intracellular PLA2 family.36,37) Several groups of PLA2 enzymes have been identified in the mammalian brain,38–41) and their specificity have been characterized in previous studies. ARA is selectively hydrolyzed by cPLA2 in a calcium-dependent manner, whereas DHA in sn-2 position is selectively hydrolyzed in a calcium-independent manner by iPLA2.42) iPLA2 and cPLA2 have been reported to be expressed in different brain regions, including astrocytes.43–45) After LPS stimulation, cPLA2 and iPLA2 mRNA level were concomitantly increased at the timing which DHA or ARA increase in the media. These results suggesting that the increasing of DHA and ARA in the media with LPS might be caused increment of intracellular FFA through increasing of intracellular cPLA2 and iPLA2.
In conclusion, we demonstrated that astrocytes release DHA and ARA during the inflammatory state to control inflammation processes in the CNS. Therefore, our findings suggest that astrocytes-derived PUFAs contribute to the maintenance of brain function and its environment and perform as one of the modulators of neuronal function under inflammatory conditions.
This work was supported by the Takeda Science Foundation and a Grant-in-Aid for Scientific Research (C) (15K10566) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The authors declare no conflict of interest.