2016 Volume 39 Issue 7 Pages 1130-1136
2016 Volume 39 Issue 7 Pages 1130-1136
The pathogenesis of Alzheimer’s disease (AD) has been linked to the deficiency of neurotransmitter acetylcholine (ACh) in the brain, and the main treatment strategy for improving AD symptoms is the inhibition of acetylcholinesterase (AChE) activity. In the present study, we aimed to identify potent AChE inhibitors from Cinnamomum loureirii extract via bioassay-guided fractionation. We demonstrated that the most potent AChE inhibitor present in the C. loureirii extract was 2,4-bis(1,1-dimethylethyl)phenol. To confirm the antiamnesic effects of the ethanol extract of C. loureirii, mice were intraperitoneally injected with the neurotoxin trimethyltin (2.5 mg/kg) to induce cognitive dysfunction, and performance in the Y-maze and passive avoidance tests was assessed. Treatment with C. loureirii extract significantly improved performance in both behavioral tests, suggesting that this extract may be neuroprotective and therefore beneficial in preventing or ameliorating the degenerative processes of AD, potentially by restoring cholinergic function.
The pathophysiology of Alzheimer’s disease (AD), a type of dementia in the elderly, is highly complex. This progressive degenerative disorder was first discovered by Dr. Alois Alzheimer in 1907 and is characterized by incapacitating memory and language losses and impairments in cognitive and behavioral functions. A neuropathological diagnosis of AD includes deposition of extracellular β-amyloid (Aβ) plaques in the cerebral cortex and hippocampus areas, accumulation of intracellular neurofibrillary tangles of abnormally phosphorylated τ, astrocytic gliosis, inflammatory cascades, and degeneration of basal forebrain cholinergic neurons.1–3)
Cholinergic neurotransmission in the central nervous system (CNS) involves release of acetylcholine (ACh) from presynaptic terminals, binding of ACh to cognate receptors on postsynaptic cells, and downstream signal transduction.4) To date, the most significant and best approach for the prevention and treatment of AD is the inhibition of acetylcholinesterase (AChE).5) AChEs are present in the synaptic cleft of cholinergic synapses and rapidly degrade ACh into choline and acetic acid.6) These enzymes decrease the concentration of ACh in the synapse and downregulate ACh-induced signaling pathways.7) Currently, many studies have focused on the use of AChE inhibitors (AChEIs) to reverse abnormally elevated AChE activity in patients with AD, thereby restoring ACh levels and ameliorating AD.8,9)
Traditionally, various edible plants have been used as medicinal remedies in Asian countries, including Korea, since they are rich sources of diverse secondary metabolites, often called phytochemicals. Notably, Cinnamomum loureirii is one of the most widely used herbal medicines in Korea. The inner bark of C. loureirii is obtained from the trees belonging to the family Lauraceae10) and is often used as a spice and flavoring agent. C. loureirii contains large amounts of bioactive molecules, including essential oils, tannin, mucus, and coumarins.11) Previous studies demonstrated that C. loureirii might be effective in the treatment of gastritis, dyspepsia, inflammatory diseases, and blood circulation disturbances and may possess anti-pyretic, analgesic, anti-allergic, and anti-ulcerogenic effects.12,13) To the best of our knowledge, however, no study has investigated the inhibitory effects of C. loureirii on AChE activity utilizing in vitro and in vivo models.
The aim of the present study was to identify AChEIs from C. loureirii. In order to isolate a candidate AChEI, the ethanol extract of C. loureirii was subjected to solvent partitioning, open column chromatography, TLC, and HPLC.14,15) The chemical structure of the purified active compound was determined using GC-MS and NMR spectroscopy. In addition, the memory enhancing effect of C. loureirii in trimethyltin (TMT)-induced cognitive dysfunction was assessed using two behavioral tests, the Y-maze test and passive avoidance test.
Roswell Park Memorial Institute (RPMI)-1640 medium, heat-inactivated horse serum (HS), fetal bovine serum (FBS), and antibiotic-antimycotic were purchased from Gibco-Invitrogen (Grand Island, NY, U.S.A.). Acetylthiocholine iodide, 5,5′-dithiobis-(2-nitro)benzoic acid (DTNB), 1,5-bis-(4-allydimethyllammoniumphenyl)-pentane-3-one dibromide (BW 284c51), tacrine (9-amino-1,2,3,4-tetrahydroacridine), dimethyl sulfoxide (DMSO), and TMT chloride were purchased from Sigma (St. Louis, MO, U.S.A.). All other chemicals used were of analytical grade purity unless otherwise specified.
Cell CultureRat pheochromocytoma (PC12; ATC C, Manassas, VA, U.S.A.) cells were cultured in RPMI-1640 medium supplemented with 10% HS (v/v), 5% FBS (v/v), and 1% antibiotic-antimycotic (v/v). Cultures were maintained at 37°C with 5% CO2 and water saturation. When the culture was 80–90% confluent, the cells were subcultured. The medium was replaced every 2 to 3 d.
Measurement of AChE ActivityAChE activity was determined using the modified spectrophotometric method of Ellman et al.16) For the enzyme source, PC12 cells were homogenized in Tris–HCl buffer [20 mM Tris–HCl (pH 7.5) containing 150 mM NaCl, 10 mM MgCl, and 0.5% Triton X-100] and then centrifuged at 10000×g for 15 min. The supernatant was used as the enzyme source, and ACh iodide was used as the reaction substrate. DTNB was selected to quantify the thiocholine produced from the enzymatic hydrolysis of AChE. Briefly, 10 µL of each sample was mixed with 10 µL of enzyme solution and added to 70 µL of reaction mixture (50 mM sodium phosphate buffer [pH 8.0] containing 0.5 mM ACh iodide and 1 mM DTNB). This reaction mix was incubated at 37°C for 15 min. The final enzyme reactions were monitored at a wavelength of 405 nm using a 96-well microplate reader (Model 550; Bio-Rad, Hercules, CA, U.S.A.). Tacrine was utilized as a reference standard in order to assess the AChE inhibitory effects of each group.
Extraction of C. loureiriiDried C. loureirii (4 kg) was purchased from Gyung-dong market, an oriental medicine store in Seoul, South Korea. The plant material was finely pulverized to powder, and 4 kg of the powder was blended with five volumes of ethanol (100 L) by shaking for 24 h at room temperature. The resulting supernatant was filtered through Whatman ashless filter paper No. 42 (GE Healthcare Life Sciences, Chalfont St. Giles, U.K.), and the residue was extracted again with fresh ethanol. This procedure was repeated five times, and the solution was completely dried using a rotary evaporator at 39°C. The percentage yield of C. loureirii extract was 26.1% (w/w).
Isolation of the AChE Inhibitor from C. loureiriiThe C. loureirii ethanol extract (998 g) was dissolved in distilled water (1560 mL), and then respectively partitioned with n-hexane (4680 mL), chloroform (4680 mL), and ethyl acetate (4680 mL), repeated three times, for 24 h. Of nine fractions, the fraction with the most pronounced inhibitory effect on AChE was chosen and subjected to 33 sub-fractions through gradient elution with a mixture of the solvents (chloroform–ethanol; 100 : 0, 90 : 10, 80 : 20, 70 : 30, 60 : 40, 50 : 50, 40 : 60, 30 : 70, 20 : 80, 10 : 90, 0 : 100, v/v, respectively, repeated three times per each elution) using gradient-elution silica-gel open column chromatography. The most potent fraction was subjected to TLC and then visualized under visible and ultraviolet light (254 and 365 nm, Vilber Lourmatm, Collégien, France). Each band separated on TLC was scraped off and extracted with ethanol and/or chloroform for the enzyme assay. Amongst all TLC bands scraped and examined, a band representing the most potent inhibitory effects against AChE was selected and subjected to HPLC analysis. Waters HPLC system (Waters Co., Milford, MA, U.S.A.) equipped with a 2690 pump, a 2996 photodiode array detector, and Empower2 software was utilized. The analytical column was a C18 µ-Bondapak™ reverse phase column (Waters Co., 3.9×300 mm, 125 Å, 10 µm). Mobile phases were employed for gradient elution with water (A) and HPLC-grade ethanol (B) under the following conditions: initially 100% A for 10 min, linear to 0% A over 50 min, followed by isocratic elution at 0% A for an additional 10 min, to 100% A over 10 min, and 100% A isocratic for 10 min at a flow rate of 0.5 mL/min. The structure of the purified active compound was then determined by GC-MS and 13C/1H-NMR spectroscopic techniques (Korea Basic Science Institute, Western Seoul Center).
AnimalsMale Institute of Cancer Research (ICR) mice (5-week-old) were purchased from DBL Co. (Chungbuk, South Korea). Mice were housed in groups of eight and maintained under a 12 h light/dark cycle at a constant temperature of 23±1°C and relative humidity of 55%. The C. loureirii ethanol extract was supplemented in commercial diet (RodFeed; DBL Co., Chungbuk, South Korea) at concentrations of 400, 800, and 1200 mg/kg body weight (wt). All animals were allowed free access to diets and water. All experimental procedures were approved by the Animal Care and Use Committee of Korea University and performed in compliance with the guideline thereof.
TMT InjectionAfter animals received the diets for 3 weeks, mice of the TMT group and sample groups received a single intraperitoneal injection of TMT. The TMT was dissolved in 0.85% (v/v) sodium chloride, and dosage was determined previously, as described (2.5 mg of TMT/kg body wt).17) The negative control group was injected with sodium chloride solution alone. The behavioral tests were conducted 1–2 d after TMT injection.
Y-Maze TestThe maze was made of black plastic with three equal angled arms (33 cm long×10 cm wide×15 cm high) in a Y shape. Each mouse was initially placed within an arm and allowed to explore the maze over 8 min. The sequence and number of arm entries visited were monitored and manually recorded. The spontaneous alternation behavior score (%) was calculated by dividing the possible alternation by the total number of arm entries minus 2, multiplied by 100. The entrance numbers per trial was used as an indicator of locomotor activity.18)
Passive Avoidance TestThe test was carried out in a chamber with two compartments (illuminated/dark) with a stainless grid floor that had electrical conductibility. For the acquisition trials, the mouse was placed in the illuminated compartment. When a mouse entered the dark chamber, an electrical foot shock (0.5 mA, 1 s) was applied through the grid floor. In the retention trial, the same procedure was performed 24 h after training, and the mouse was again placed in the illuminated compartment. The time required to enter the dark chamber was recorded up to 5 min.19)
Statistical AnalysisData are presented as mean±standard deviation (S.D.). Statistical analyses were performed using the Statistical Analysis System software package (Cary, NC, U.S.A.). Significant differences were calculated by one-way ANOVA followed by the Scheffe’s multiple range test. A p value less than 0.05 was considered statistically significant.
The C. loureirii ethanol extract (total weight: 998 g) was subjected to liquid–liquid partition. Among the nine fractions obtained, the second fraction of chloroform layer was selected for further isolation since it exhibited the highest inhibitory activity against AChE relative to the other layers (Fig. 1). This chloroform extract (total weight: 11.9 g) was then partitioned into 33 fractions by open column chromatography, and these fractions were tested for their ability to inhibit AChE. Among all the fractions, the fifth fraction showed the highest inhibitory effect on AChE (Fig. 2). This fraction (total weight: 4.5 g) was then subjected to TLC to separate the active compound. The bands clustered near the intermediate range of Rf value, i.e., 0.09–0.92, and these bands were extracted with ethanol and/or chloroform. Of all the TLC bands scraped, the ninth band (Rf value=0.64) showed the most effective inhibitory effect against AChE activity (Fig. 3), and hence, it subsequently was subjected to HPLC (total weight: 128 mg). As shown in Fig. 4, a significant single peak was seen at 31.43 min of the chromatogram. The single peak was collected at the retention time depending on the chromatography seen, and the final yield of the isolated component from C. loureirii (4 kg) was about 7 mg.
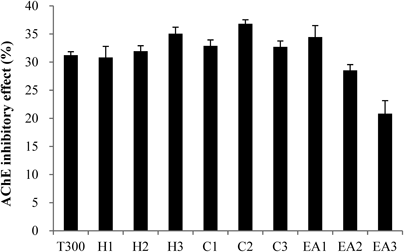
The value (%) of AChE activity for each fraction was calculated relative to control activity (100%). The T300 was treated with 300 nM tacrine, a known specific AChE inhibitor. The sample groups were fractionated with hexane (H1–H3), chloroform (C1–C3), and ethyl acetate (EA1–EA3), respectively, repeated 3 times per each elution. The sample concentration was 1 mg/mL. Each value represents the mean±standard deviation (S.D.) (n=4).
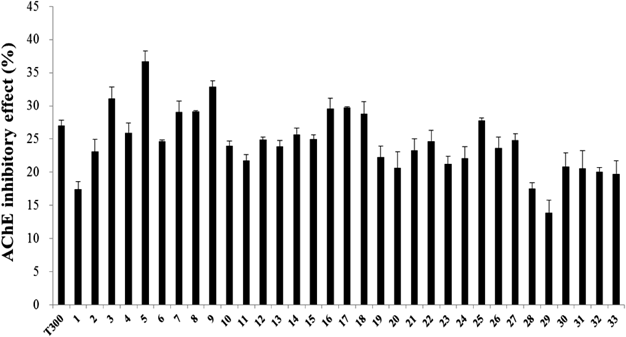
The value (%) of AChE activity for each fraction was calculated relative to control activity (100%). The T300 was treated with 300 nM tacrine, a known specific AChE inhibitor. Second chloroform fraction of C. loureirii extract was fractionated by gradient elution with a mixture of chloroform and ethanol (100 : 0, 90 : 10, 80 : 20, 70 : 30, 60 : 40, 50 : 50, 40 : 60, 30 : 70, 20 : 80, 10 : 90, 0 : 100, v/v, respectively, repeated 3 times per each elution). The sample concentration was 1 mg/mL. Each value represents the mean±S.D. (n=4).
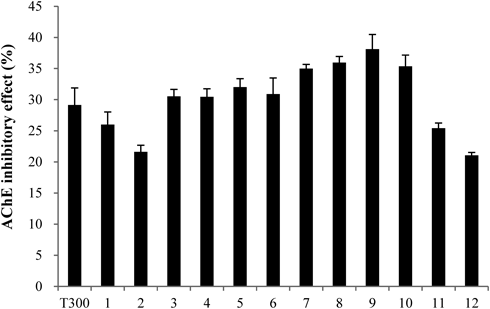
The value (%) of AChE activity for each fraction was calculated relative to control activity (100%). The T300 was treated with 300 nM tacrine, a known specific AChE inhibitor. Fifth fraction of C. loureirii extract were separated as well-defined bands that clustered near the intermediate range of Rf value, i.e., 0.09–0.92. The sample concentration was 1 mg/mL. Each value represents the mean±S.D. (n=4).
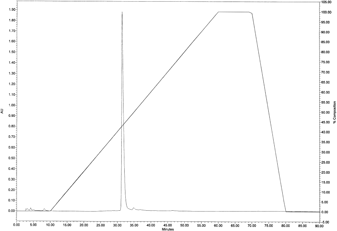
C. loureirii extract was analyzed by HPLC with UV detection at 290 nm. The analysis was performed with a C18 µ-bondapak™ column (reverse phase column, size: 3.9×300 mm) at 35°C. The extract was eluted (eluent A: water, eluent B: ethanol) at a flow rate of 0.5 mL/min, and the gradient changes from 0 to 100% B over 50 min. Injection volume was 20 µL and retention time was 31.43 min.
The structure of the candidate AChEI was elucidated by GC-MS and 13C/1H-NMR and was identified as a 2,4-bis(1,1-dimethylethyl)phenol (BP) (m/z=206), which is a natural polyphenol with a phenolic structure (Figs. 5, 6).
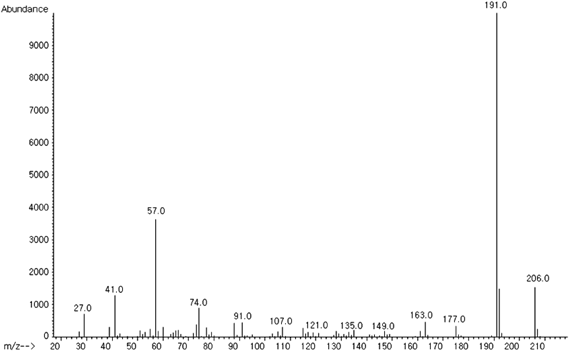
The sample was dissolved in ethanol and then subjected to the GC-MS system. The spectrum was recorded on positive ion GC-MS (JMS-AX505WA, JEOL, Akishima, Japan). The molecular ion (m/z 206) and methyl cleavage ion (m/z 191) were shown in mass spectrum. Also, the identification of 2,4-bis(1,1-dimethylethyl)phenol was confirmed by the NIST library search.
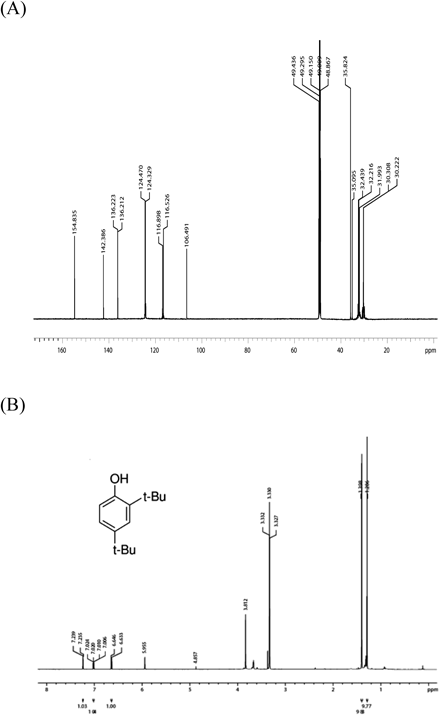
13C-NMR spectrum (A) and 1H-NMR spectrum (B) were obtained with a high resolution NMR spectrometer (Avance-600; Bruker, Germany) operating at 600 MHz and 25°C.
The AChE inhibitory activity of BP was tested. As shown, AChE inhibitory activity was increased by the BP with a dose-dependent manner (Table 1).
| Concentration of BP | AChE inhibitory activity (%) |
|---|---|
| 10 mM | 17.12±1.33 |
| 20 mM | 21.19±1.39 |
| 40 mM | 30.45±3.41 |
The value (%) of AChE activity for each fraction was calculated relative to control activity (100%). Each value represents the mean±standard deviation (S.D.) (n=4).
The effect of C. loureirii extract on immediate spatial working memory was investigated using the Y-maze test (Fig. 7A). The TMT-treated group showed a significant deficit (17.2% decrease) in spontaneous alternation behavior compared to the negative control group. However, dietary supplementation of C. loureirii extract significantly increased the alternation behavior compared with that of the TMT-treated group. Even though the C. loureirii extract efficiently restored spatial working memory in the Y-maze test, the total number of arm entries was not significantly different among all the groups, suggesting that TMT and the samples affected nonspecific processes, including arousal, attention, or sensory motor functions (Fig. 7B).
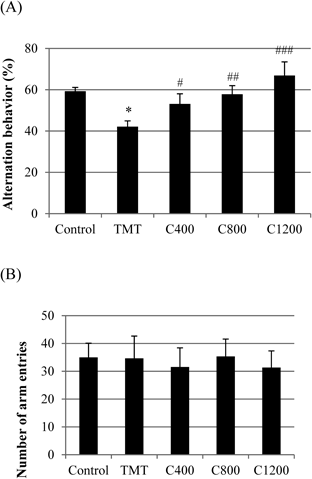
The negative control group was injected with sodium chloride solution. The TMT group received a single intraperitoneal injection of TMT (2.5 mg/kg body wt). All sample groups (C400, C800, C1200) received a single intraperitoneal injection of TMT after 3 weeks of dietary supplementation. Commercial diet was supplemented with C. loureirii extract at concentrations of 400, 800, and 1200 mg of C. loureirii extract/kg body wt. The spontaneous alternation behaviors (A) and the number of arm entries (B) were measured for 8 min. Each value represents the mean±S.D. for 8 mice. (* p<0.05 as compared with that of the negative control group, # p, ## p and ### p<0.05 as compared with that of the TMT group).
Learning and short-term memory impairment of TMT-treated mice were assessed by the passive avoidance test (Fig. 8). The step-through latency of the TMT group was 67.2% less than that of the negative control group (i.e., TMT-free control group), and the administration of C. loureirii extract significantly improved this reduction in TMT treated animals. Importantly, TMT treatment impaired retention of the single-trial passive avoidance test. Furthermore, mice treated with TMT and samples did not exhibit any significant differences in body or brain weight and acute liver toxicity when compared to control mice (data not shown). Thus, improvements in memory performance with C. loureirii were not due to changes in weight or levels of serum aminotransferases.
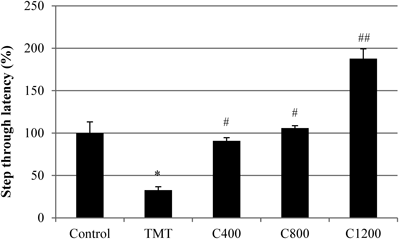
The negative control group was injected with sodium chloride solution. The TMT group received a single intraperitoneal injection of TMT (2.5 mg/kg body wt). All sample groups (C400, C800, C1200) received a single intraperitoneal injection of TMT after 3 weeks of dietary supplementation. Commercial diet was supplemented with C. loureirii extract at concentrations of 400, 800, and 1200 mg of C. loureirii extract/kg body wt. The step-through latency was measured over 5 min. Each value represents the mean±S.D. for 8 mice (* p<0.05 as compared with that of the negative control group, # p and ## p<0.05 as compared with that of the TMT group).
In order to isolate a potent AChEI from natural sources, often considered more safe, the ethanol extract of C. loureirii was subjected to bioassay guided purification: solvent partitioning, open column chromatography, TLC, and HPLC.17) The structure of a candidate compound was elucidated using GC-MS and NMR; the structure of the purified compound was determined as BP. In our subsequent in vitro assay, this constituent inhibited the AChE activity with a dose dependent manner. Previously, it was reported that this phenolic compound is present in wild plants and mushrooms.20–22) Of note, BP has been widely used as a raw material for phosphite antioxidants and as an intermediate for the preparation of ultraviolet stabilizers as well.23) According to previous reports, BP is an antioxidant with remarkable cytotoxic activity against cancer cells,24,25) and it has been shown to decrease the degree of low-density lipoprotein (LDL)-oxidation.26) Moreover, BP was reported to be the active compound in the ethanol extract of Ipomoea batatas that was shown to be protective against Aβ-induced neurotoxicity.27)
TMT, a potent neurotoxicant, has been used to induce experimental models of AD.28) The administration of TMT in rodents has been reported to cause neuropathological and behavioral alternations, including aggression, tremor, and hyperexcitability.29) These changes were correlated with degeneration of cholinergic axons and changes in ACh levels and their receptors.30) Kobayashi et al. founded that TMT toxicity causes a decrease of ACh release in cerebral tissue.31) These changes might be due to hippocampal loss of neurons, leading to deficits in learning and memory. As shown in Figs. 7A and 8, supplementation of C. loureirii extract to TMT-treated mice attenuated TMT-induced impairment in a dose-dependent manner. Thus, C. loureirii extract possibly improves learning and memory function in TMT-treated animals by inhibiting AChE thereby restoring ACh levels.
Co-administration of C. loureirii extract and TMT had no effect on the body weight or brain weight of mice. In addition, we found that C. loureirii extract was not hepatotoxic and exerted no adverse effect on liver function. Future investigation might be needed to examine the effect of exposure to BP on TMT-induced memory deficiency in vivo; further, it would be interesting to compare the biological potency between crude C. loureirii extract and the purified constituent, BP.
This study demonstrated that C. loureirii extract inhibited AChE in vitro and was neuroprotective against cognitive dysfunction resulting from TMT exposure. Overall, C. loureirii, including bioactive polyphenol, is a potent source of AChEIs that might be beneficial in slowing or preventing the degenerative processes of AD by restoring the cholinergic basal forebrain system.
This research was supported by High Value-Added Food Technology Development Program, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
The authors declare no conflict of interest.