2016 Volume 39 Issue 7 Pages 1137-1143
2016 Volume 39 Issue 7 Pages 1137-1143
Visceral obesity induces the onset of metabolic disorders such as insulin resistance and diabetes mellitus. Adipose tissue is considered as a potential pharmacological target for treating metabolic disorders. The fruit of Terminalia bellirica is extensively used in Ayurvedic medicine to treat patients with diseases such as diabetes mellitus. We previously investigated the effects of a hot water extract of T. bellirica fruit (TB) on obesity and insulin resistance in spontaneously obese type 2 diabetic mice. To determine the active ingredients of TB and their molecular mechanisms, we focused on adipocyte differentiation using mouse 3T3-L1 cells, which are widely used to study adipocyte physiology. We show here that TB enhanced the differentiation of 3T3-L1 cells to mature adipocytes and that one of the active main components was identified as gallic acid. Gallic acid (10–30 µM) enhanced the expression and secretion of adiponectin via adipocyte differentiation and also that of fatty acid binding protein-4, which is the target of peroxisome proliferator-activated receptor gamma (PPARγ), although it does not alter the expression of the upstream genes PPARγ and CCAAT enhancer binding protein alpha. In the PPARγ ligand assay, the binding of gallic acid to PPARγ was undetectable. These findings indicate that gallic acid mediates the therapeutic effects of TB on metabolic disorders by regulating adipocyte differentiation. Therefore, TB shows promise as a candidate for preventing and treating patients with metabolic syndrome.
Visceral obesity induces the onset of metabolic syndrome symptoms such as insulin resistance, diabetes mellitus, abnormal lipid metabolism, and hypertension. Adipose tissue is an endocrine organ that secretes various adipocytokines associated with the accumulation of visceral fat and insulin sensitivity.1) The adipocytes of obese individuals become hypertrophic with a diminished ability to secrete adiponectin, which enhances insulin sensitivity. Adipose tissue plays a major role in mediating the actions of insulin, and is therefore considered a potential pharmacological target for treating the symptoms of metabolic syndrome.
Thiazolidinediones are antidiabetic agents that stimulate adipocyte differentiation and enhance insulin sensitivity,2,3) however they cause adverse effects such as unexpected weight gain, edema, and hepatotoxicity.4) Therefore, it is important to investigate alternative sources of more efficacious and safer therapeutics. Recently, a number of natural products were discovered that affect adipocyte differentiation5–11) and are therefore attracting increased attention.
The fruit of Terminalia bellirica (GAERTN.) ROXB. is extensively used in Ayurvedic medicine in India and neighboring countries. Although the fruit of T. bellirica is used to treat various ailments, including diabetes mellitus,12–14) the mechanism is unknown. T. bellirica extract has the effects as follows: hypoglycemic,15–17) hypolipidemic,18–20) antihypertensive,21) antioxidant,22,23) and antiobesity.24) Therefore, it is reasonable to speculate that T. bellirica will prevent the symptoms of metabolic syndrome.
We previously investigated the preventive effect of a hot water extract of T. bellirica fruit (TB) on obesity and various metabolic disorders of type 2 diabetic mice that develop spontaneous obesity and found that treatment with TB prevents visceral fat accumulation and insulin resistance.24) Because these findings indicate that TB would directly act on adipocytes, we focused on adipocyte differentiation using mouse 3T3-L1 cells, which are widely used to study adipocyte physiology, to determine the active ingredients of TB and their molecular mechanisms.
A hot water extract of T. bellirica fruit (TB-001) was supplied by Toyo Shinyaku Co., Ltd. (Saga, Japan). A voucher specimen of TB (TB-001) was deposited at the Research Institute of Pharmaceutical Sciences, Musashino University (Tokyo, Japan). TB contains gallic acid (10.40%), ellagic acid, and tannic acid. A gallic acid standard (purity >98%) was purchased from LKT Laboratories, Inc. (St. Paul, MN, U.S.A.). Troglitazone was purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). The mouse 3T3-L1 preadipocyte cell line was purchased from Health Science Research Resources Bank (Osaka, Japan). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin (10000 unit/mL)/streptomycin (10000 mg/mL), and trypsin/ethylenediaminetetraacetic acid (EDTA) were purchased from Invitrogen (CA, U.S.A.). Isobutylmethylxanthine (IBMX), dexamethasone (DEX), insulin (INS), and Oil Red O were purchased from Sigma-Aldrich Japan (Tokyo, Japan).
Isolation and Identification of the Active Components of TBEach fraction used in this study was obtained as previously described.24) In brief, 16 g of TB was extracted three times with methanol. The methanol extract (9.23 g) was subjected to normal-phase silica gel column chromatography using a mobile phase containing chloroform, methanol, and distilled water [CHCl3–MeOH–H2O 30 : 7 : 0.5 (1125 mL)→30 : 10 : 0.5 (1012 mL)→30 : 12 : 0.8 (1070 mL)→30 : 15 : 1 (920 mL)→30 : 20 : 1 (1275 mL)→6 : 4 : 1 (1100 mL)→MeOH] and 11 fractions (Frs. 1–11) (Fr. 1: 72.7 mg, Fr. 2: 72.1 mg, Fr. 3: 215.7 mg, Fr. 4: 995.8 mg, Fr. 5: 876.5 mg, Fr. 6: 604.1 mg, Fr. 7: 1446.1 mg, Fr. 8: 680.3 mg, Fr. 9: 1000.0 mg, Fr. 10: 225.0 mg, Fr. 11: 610.0 mg) were obtained. The most active fractions for adipocyte differentiations were further fractionated and the main component was identified using TLC. The main component was characterized and quantified using 1H- and 13C-NMR and high resolution electron ionization mass spectrometry (HR-EI-MS). 1H- and 13C-NMR spectra were recorded using a JEOL JNM-AL-400 (1H 400 and 13C 100 MHz) spectrometer in DMSO-d6 and CD3OD with TMS as internal standard. The MS spectra were obtained using a JEOL JMS-700 (see Supplementary Materials).
Cell Culture3T3-L1 preadipocytes were maintained in DMEM containing 10% FBS and 1% penicillin/streptomycin at 37°C in a humidified atmosphere containing 5% CO2. In a differentiation assay, 3T3-L1 cells were seeded at a density of 3×104 cells/mL in 12-well plates. Two days after the cells reached confluence (designated day 0), the medium was replaced with induction medium supplemented with 0.25 µM DEX, 0.5 mM IBMX, and 1 µg/mL INS. After 2 d, the medium was replaced with DMEM containing 10% FBS and 1% penicillin/streptomycin medium supplemented with 1 µg/mL INS. This medium was then replaced with DMEM containing 10% FBS and 1% penicillin/streptomycin 2 d later and replaced every 2 d thereafter. Test compounds were added to cell cultures from days 0 to 4. Troglitazone (10 µM) was used as a positive control. Each test compound was dissolved in medium (TB, Frs. 4–11, gallic acid) or dimethyl sulfoxide (DMSO) (Frs. 1–3, troglitazone) and filtrated. After dilution at each concentration using medium, final DMSO concentration in Frs. 1–3 was 0.2% (v/v).
Cell Viability AssayThe maximum concentration of TB and gallic acid used in this study were determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. 3T3-L1 cells were seeded at a density of 5×104 cells/mL in 24-well plates. After 24 h, cells were treated with TB at concentrations between 0 and 100 µg/mL or with gallic acid at concentrations between 0 and 100 µM for 96 h.
Measurement of Intracellular TriglyceridesOn day 8, 3T3-L1 cells were washed twice with phosphate-buffered saline (PBS) and scraped into 150 µL of PBS containing 1% Triton X-100. The cell suspension was homogenized using a sonicator, and the triglyceride concentration was determined using Triglyceride E-Test Wako (Wako Pure Chemical Industries, Ltd., Osaka, Japan). Intracellular triglyceride content was normalized to protein content that was determined using the Lowry method.
Oil Red O StainingAfter differentiation for 8 d, cells were washed twice with PBS, fixed with 10% formalin for 10 min, and washed twice with PBS. Cells were stained with Oil Red O for 20 min, washed twice with PBS, and then photographed.
Adiponectin SecretionThe supernatant medium using days 6–8 was collected at 8 d after differentiation, and adiponectin in the medium was measured using a Mouse Adiponectin/Acrp30 assay (R&D Systems, Minneapolis, MN, U.S.A.).
Analysis of mRNA ExpressionAfter differentiation for 4 and 8 d, total RNA from adipocytes cultured in 24-well plates was isolated using the Tissue Total RNA Mini Kit (Favorgen Biotech Corp., Ping-Tung, Taiwan) in accordance with the manufacturer’s directions. Total RNA (500 ng) was used as template to synthesize cDNA in a reaction mixture (final volume, 10 µL) using a PrimeScript RT reagent Kit (TaKaRa Bio Inc., Shiga, Japan) according to the manufacturer’s directions. Quantification of gene expression was measured using a real-time PCR system (Mini Opticon, Bio-Rad Laboratories Inc., Hercules, CA, U.S.A.). PCR reaction mixtures (final volume, 20 µL) were prepared using SYBR Premix Ex Taq (TaKaRa). Cycling conditions were 30 s at 95°C, 40 cycles at 95°C (5 s each), 60°C (30 s), followed by melting curve analysis. Gene expression levels were normalized to those of β-actin mRNA.
The PCR primer sequences were as follows: β-Actin (ACTB); Forward: 5′-CCA TCC TGC GTC TGG ACC TG-3′, Reverse: 5′-TTC CCT CTC AGC TGT GGT GG-3′. Peroxisome proliferator-activated receptor gamma: PPARγ; Forward: 5′-TGA ACG TGA AGC CCA TCG AG-3′, Reverse: 5′-CTT GGC GAA CAG CTG AGA GG-3′. CCA AT enhancer binding protein alpha: C/EBPα; Forward: 5′-CGC AAG AGC CGA GAT AAA GC-3′, Reverse: 5′-CAC GGC TCA GCT GTT CCA-3′. Fatty acid binding protein 4: Fabp4; Forward: 5′-CAT GAT CAT CAG CGT AAA TGG G-3′, Reverse: 5′-TCA TAA CAC ATT CCA CCA CCA GC-3′. Adiponectin; Forward: 5′-GGC TCT GTG CTC CTC CAT CT-3′, Reverse: 5′-AGA GTC GTT GAC GTT ATC TGC ATA G-3′.
PPARγ Ligand AssayThe PPARγ ligand activity of gallic acid was determined using a RCAS PPARγ Kit (EnBio Tec Laboratories Co., Ltd., Tokyo, Japan), in accordance with the manufacturer’s instructions. This cell-free assay system uses nuclear receptors and coactivator cAMP response element binding protein (CBP). When PPARγ undergoes a ligand-induced conformational change, it binds to immobilized CBP, and this complex is detected using an ELISA. Experiments were performed in duplicate.
Statistical AnalysisData are expressed as the mean±standard deviation (S.D.). Statistical analysis was performed using Dunnett’s multiple comparison test. A p values <0.05 indicate statistical significance.
To determine the maximum TB concentration used in this study, we cultured preconfluent 3T3-L1 preadipocytes in the presence and absence of TB. TB (12.5 µg/mL) did not affect cell viability compared with the control (Fig. 1A). However, treatment with 25 µg/mL TB inhibited cell viability by approximately 16% (p<0.01). Accordingly, the maximum TB concentration used was 10 µg/mL.
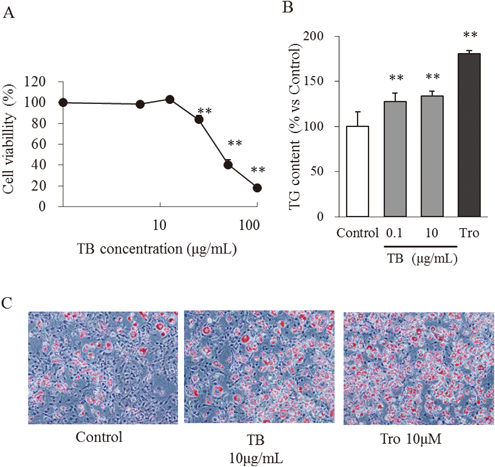
(A) Viability of cells treated with TB for 96 h. The data represent the mean±S.D. (n=4). ** p<0.01, significant difference from the control. (B) Triglyceride (TG) content of 3T3-L1 cells on day 8. Data represent the mean±S.D. (n=6). ** p<0.01, significant difference from the control. (C) Oil Red O staining was performed on day 8. Each images depicts 3T3-L1 cells treated with 0 (Control) and 10 µg/mL TB or 10 µM troglitazone (Tro).
To evaluate the effect of TB on adipocyte differentiation, 3T3-L1 preadipocytes were treated with TB for 4 d (days 0 to 4). To quantify the adipogenic effects of TB, we determined the amounts of accumulated intracellular triglycerides on day 8. Triglyceride content was significantly increased in cells treated with 0.1 and 10 µg/mL TB. (Fig. 1B). Macroscopic observations of cells stained with Oil Red O on day 8 are shown in Fig. 1C. TB (10 µg/mL) enhanced morphological changes and the accumulation of oil droplets in the same manner as troglitazone (10 µM). These results suggest that TB enhances the differentiation of 3T3-L1 preadipocytes.
Isolation and Identification of the Active Components of TBSilica gel column chromatography of the methanol extract of TB yielded 11 fractions (Frs. 1–11) with the yields (w/w) as follows: 0.45, 0.45, 1.4, 6.2, 5.5, 3.8, 9.0, 4.2, 6.2, 1.4, and 3.8%, respectively. To identify the component(s) that induced differentiation, 0.1, 1, and 10 µg/mL of each fraction was added to cultures of 3T3-L1 cells for 4 d. On day 8, Fr. 1, Fr. 2, Frs. 4–9 significantly increased the accumulation of intracellular triglycerides compared with the control (Fig. 2). Fractions 4 and 5 were the most active fractions (77 and 94% increases in intracellular triglyceride content, respectively, compared with untreated cells). Using TLC, Frs. 4 and 5 were confirmed to consist of a single component (data not shown). Using 1H- and 13C-NMR and MS, we identified gallic acid as a major species present in Frs. 4 and 5 (Supplementary Materials).

Triglyceride (TG) content of 3T3-L1 cells 8 d after the induction of differentiation. The data represent the mean±S.D. (n=3). * p<0.05, ** p<0.01, significant differences from the control.
Treating preconfluent 3T3-L1 preadipocytes with 30 µM gallic acid had no detectable effect on cell viability compared with the control. However, the viability of 3T3-L1 preadipocytes treated with gallic acid (60, 100 µM) was significantly inhibited (Fig. 3A). Accordingly, the maximum gallic acid concentration used in the present study was 30 µM.

(A) Viability of 3T3-L1 cells after treatment with gallic acid for 96 h. Data represent the mean±S.D. (n=4). ** p<0.01, significant difference from the control. (B) Triglyceride (TG) of 3T3-L1 cells treated with gallic acid or troglitazone (Tro). Data represent the mean±S.D. (n=4). * p<0.05 and ** p<0.01, significant differences from the control. (C) Oil Red O staining was performed on day 8. Each images depicts 3T3-L1 cells treated with 0 (Control), 3, 10 and 30 µM gallic acid.
To evaluate its effect on adipocyte differentiation, 3T3-L1 preadipocytes treated with 3, 10, and 30 µM gallic acid for 4 d. On day 8, the intracellular triglyceride content of cells treated with 10 and 30 µM gallic acid was significantly and a dose dependently increased compared with the control (Fig. 3B). As shown in Fig. 3C, the treatment of gallic acid increased differentiated adipocytes in a dose-dependent manner.
Adiponectin SecretionEight days after induction of differentiation, gallic acid enhanced the secretion of adiponectin in a dose-dependent manner (Fig. 4A). Treatment with 10 and 30 µM gallic acid significantly enhanced secretion of adiponectin compared with the control.

(A) Concentrations of adiponectin secreted from 3T3-L1 cells 8 d after the induction of differentiation. The data represent the mean±S.D. (n=4–5). Troglitazone (Tro: 10 µM). (B, C) Expression of mRNAs encoding Fabp4 and adiponectin on 8 d after the induction of differentiation, respectively. Data represent the mean±S.D. (n=9–10). (D, E) Expression of mRNAs encoding PPARγ and C/EBPα by 3T3-L1 cells 4 and 8 d after induction of differentiation, respectively. Data represent the mean±S.D. (control and gallic acid; n=7–10, 10 µM Tro; n=3). * p<0.05 and ** p<0.01, significant difference from the control.
On day 8, treatment of 3T3-L1 cells with 10 and 30 µM gallic acid significantly increased the expression of Fabp4 mRNA, a marker of adipocyte differentiation as well as that of adiponectin (Figs. 4B, C). In contrast, on days 4 and 8, gallic acid did not alter the expression of mRNAs encoding PPARγ and C/EBPα, the key regulators of adipocyte differentiation unlike troglitazon (Figs. 4D, E).
PPARγ Ligand AssayGallic acid did not alter the expression of the mRNA encoding PPARγ, despite enhanced expression of the PPARγ target genes Fabp4 and adiponectin. We next determined whether gallic acid acts as a ligand of PPARγ. Gallic acid binding to PPARγ was negligible at any concentration (Fig. 5), and gallic acid showed no activity for binding to PPARγ even if increasing concentrations up to 100 µM (data not shown). Meanwhile, troglitazone, which was used as a positive control, showed a strong and dose-dependent activation.
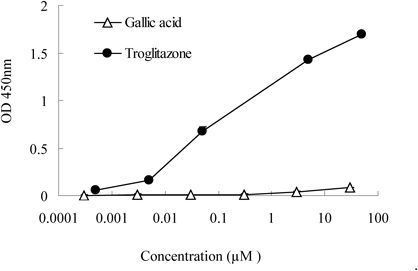
The PPARγ ligand activity of gallic acid was measured using a nuclear receptor cofactor assay system. Troglitazone served as the positive control.
In the present study, we isolated gallic acid from a hot water extract of T. bellirica fruit and identified it as the component with the greatest effect on the differentiation of the mouse 3T3-L1 preadipocyte cell line. In our previous study, we found that TB prevented obesity and various metabolic disorders in spontaneously obese type 2 diabetic mice.24) In particular, TB prevented the accumulation of visceral fat, improved glucose intolerance, and decreased homeostasis model assessment of insulin resistance (HOMA-IR) in these mice, and we therefore focused on adipocytes as a target of TB. We show here that TB enhanced the differentiation of 3T3-L1 preadipocytes in a similar manner to that of the positive control troglitazone. We also indicated that gallic acid enhanced the expression and secretion of adiponectin in adipocytes. Our present results indicate that T. bellirica including gallic acid is therefore a potential drug candidate for enhancing adiponectin secretion via enhancing the differentiation of adipocytes.
T. bellirica was reported to contain gallic acid, galloyl glucose, chebulagic acid, ellagic acid,25,26) β-sitosterol, ethylgallate, sugars,25) bellericanin,27) lignans, and flavan.28) Activity-guided fractionation of the TB methanol extract revealed that TB comprises multiple active compounds. We isolated one main active component and identified it as gallic acid. Moreover, a gallic acid standard similarly enhanced the differentiation of adipocytes in a dose-dependent manner. Although the amounts of Frs. 1 and 2 represented 0.45% of the content of TB, we were unable to isolate active components. Because of its content and activity, it is reasonable to conclude that gallic acid is the major component of TB that enhances the differentiation of adipocytes.
Gallic acid is a naturally abundant phenolic compound and is active as an antioxidant as well as an anticancer,29) and antiobesity agent.30) Interestingly, our present study shows that a low concentration of gallic acid (10–30 µM) enhanced the differentiation of adipocytes and a high concentration (>60 µM) inhibited their viability. The latter result is consistent with that of another study showing that gallic acid inhibits cell population growth of 3T3-L1 preadipocytes and induces these cells to undergo apoptosis,31) suggesting that T. bellirica including gallic acid acts through several mechanisms. For the first time to our knowledge, we show here that at relatively low concentrations (10, 30 µM), gallic acid enhanced adipocyte differentiation.
There is limited information about the effects of orally administered gallic acid. For example, a study of the pharmacokinetics of gallic acid in humans and its relative bioavailability from tea found that the maximum plasma concentration of gallic acid after oral administration of 50 mg is 1.8 µM.32) The maximum concentration of gallic acid in the portal vein and inferior vena cava after oral administration of 100 mg/kg of gallic acid to rats is approximately 30 µM.33) Considering the gallic acid content of TB, the dose of gallic acid administrated to spontaneously obese type 2 diabetic mice in our previous study is comparable with a dose of 100–300 mg/kg.24) Therefore, it is reasonable to assume that the gallic acid concentrations (10–30 µM) used here may be consistent with those in mice that were the subject of our previous study.24)
We show here that gallic acid enhanced the expression of the mRNA encoding the marker of adipocyte differentiation Fabp4. Further, gallic acid enhanced the expression and secretion of adiponectin. Adiponectin is an adipose-specific cytokine to regulate insulin sensitivity. Insulin resistance in obesity is linked to the down-regulation of adiponectin, and the replenishment of adiponectin improves insulin resistance in obesity mouse models.34) In our study, it was suggested that gallic acid may improve insulin sensitivity, which confirmed the previous experiment on spontaneously obese type 2 diabetic mice24) via enhancing the differentiation of adipocytes that secrete adiponectin.
Adipocytes differentiation is mainly controlled by the transcription factors PPARγ and C/EBPα,35) which are mutually activated and promote the expression of adipose-specific genes that encode proteins mediating adipose-specific functions. The antidiabetic thiazolidinediones are potent and specific ligands for PPARγ that stimulate the activity and expression of PPARγ.35–37) However, it was reported that thiazolidinediones caused edema depending on the ligand-binding ability of PPARγ as adverse effect.38) In contrast, the binding of gallic acid to PPARγ was undetectable in this study, which is consistent with the results of another report,39) suggesting that gallic acid could be a safer therapeutic than thiazolidinediones in adverse effects including edema. In this study, gallic acid enhanced the differentiation but had no effect on the expression of the mRNAs encoding PPARγ and C/EBPα. It is suggested that gallic acid enhances adipocytes differentiation via a PPARγ-independent mechanism. Recently, adipocyte differentiation has been reported to be epigenetically regulated via methylation and acetylation of histone. There are several natural polyphenols which have the ability to modulate transcription by altering the chromatin structure via modification of histones. Several compounds were reported to have potent histone acetyltransferase and histone deacetylase (HDAC) inhibitory activities.40,41) Hsu et al. reported that gallic acid inhibits HDAC activity in 3T3-L1 preadipocytes.42) Although further investigation is required to determine how gallic acid enhances adipocyte differentiation, the inhibition of HDAC activity may contribute in the enhancement of adipocyte differentiation.
In summary, we show here that gallic acid is one of the active constituents of TB and that it enhanced the expression and secretion of adiponectin in adipocytes. These findings serve as a foundation for further investigations on the potential of gallic acid and TB to prevent and treat metabolic syndrome.
This work was supported by JSPS KAKENHI Grant Numbers 19590703 and 26861860.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.