2016 Volume 39 Issue 7 Pages 1220-1223
2016 Volume 39 Issue 7 Pages 1220-1223
It is well known that Langerhans cells (LCs) work as the primary orchestrators in the polarization of the immune milieu towards a T helper type 1 (Th1) or T helper type 2 (Th2) response. In this study, we investigated the effects of tacrolimus and betamethasone, each used as topical applications in atopic dermatitis (AD), on Th2 cell development mediated by LCs. LC-like dendritic cells (LDCs) were generated from mouse bone marrow cells and used as substitutes for LCs. Mice were primed with ovalbumin (OVA) peptide-pulsed LDCs, which had been treated with tacrolimus or betamethasone, via the hind footpad. After 5 d, the cytokine response in the popliteal lymph nodes was investigated by enzyme-linked immunosorbent assay. The expression of cell surface molecules on LDCs was investigated via reverse transcriptase polymerase chain reaction. Administration of OVA peptide-pulsed LDCs, which had been treated with betamethasone, inhibited Th2 cell development, as represented by the down-regulation of interleukin-4 production, and also inhibited Th1 cell development, represented by the down-regulation of interferon-γ production. However, tacrolimus-treated LDCs did not induce such inhibition of the development of Th1 and Th2 cells. The inhibition of Th1 and Th2 cell development was associated with the suppression of CD40 and T-cell immunoglobulin, and mucin domain-containing protein (TIM)-4 expression, respectively, in LDCs. These results suggest that the topical application of betamethasone to skin lesions of patients with AD acts on epidermal LCs, and may inhibit the development of Th2 cells, thus being of benefit for the control of AD.
Atopic dermatitis (AD) is a chronic inflammatory skin disease with immunopathologic features that vary depending on the duration of the lesions. The majority of AD patients show increased expression of T helper type 2 (Th2) cytokines such as interleukin (IL)-4, IL-5 and IL-13 in their peripheral blood mononuclear cells.1) Langerhans cells (LCs) are a subpopulation of the bone marrow-derived dendritic cells (DC). They are antigen-presenting cells (APCs), capable of internalizing and processing antigen.2) Because they reside in epithelium of skin they may be the primary target cells for antigens entering the skin.3) After antigen uptake, LCs migrate to regional lymph nodes where peptides, in the context of major histocompatibility complex (MHC) class II molecules, are presented to naïve Th cells bearing appropriate Th cell receptors. This initial signal delivered to naïve Th cells, together with a second signal, delivered in part by interaction between the CD80 and CD86 molecules on LCs and CD28 on Th cells, results in activation of the Th cells.4,5) Furthermore, LCs work as the primary orchestrators in the polarization of immune responses towards a Th1 or a Th2 immune response. The nature of the polarization is influenced by a number of factors and, in particular, the development of Th2 cells, producing type II cytokines such as IL-4, IL-5 and IL-13, plays a pivotal role in inducing allergic inflammation.6) Therefore, allergic inflammation might be controllable through regulation of LCs. In our previous study, we succeeded in generating LC-like dendritic cells (LDCs) from murine bone marrow cells as substitute cells for LCs, and through them developed Th1 cells or Th2 cells.7) Although tacrolimus and betamethasone are widely used for topical application in AD, it is unclear whether they inhibit Th2 cell development through LCs. In the present study, therefore, we investigated the effects of tacrolimus and betamethasone on LDCs.
Specific-pathogen-free BALB/c (wild-type) mice and DO 11.10 TCR Tg mice (ovalbumin (OVA)323–339-specific I-Ad-restricted T cell receptor (TCR)-transgenic mice) were obtained from Japan SLC (Hamamatsu, Japan) and The Jackson Laboratory (Bar Harbor, Maine, U.S.A.), respectively, and used at the age of 6 to 8 weeks. They were housed in plastic cages with sterilized paper bedding in a clean, air-conditioned room at 24°C and allowed free access to a standard laboratory diet and water. All procedures performed on the mice were in accordance with the Guidelines of the Animal Care and Use Committee of Meiji Pharmaceutical University, Tokyo.
Th1 Cell and Th2 Cell Regulation by Tacrolimus- or Betamethasone-Treated LDCsThe preparation and culture of BALB/c mouse bone marrow cells to generate LDCs were performed according to the method established in our previous study.7) Th1/Th2 regulation by LDCs was investigated by modifying the method used in our previous study.7) Briefly, LDCs were adjusted to 2×105 cells/mL in RPMI 10 (RPMI 1640 medium with L-glutamine (Sigma-Aldrich, St. Louis, MO, U.S.A.) containing 10% fetal bovine serum (Sigma-Aldrich), 25 mM N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES) (Sigma-Aldrich), 100 U/mL penicillin and 100 µg/mL streptomycin (Gibco RBL, Grand Island, NY, U.S.A.)) and then incubated with 6 µM OVA peptide (323-ISQAVHAAHAEINEAGR-339; obtained from Operon Biotechnologies, Tokyo, Japan) in the absence or presence of 1–10 µM tacrolimus (gift from Astellas Pharma, Tokyo, Japan) or betamethasone (Tokyo Chemical Industry, Tokyo, Japan) at 37°C in a humidified atmosphere with 5% CO2. The cells were collected after incubation for 18 h, washed in RPMI 10, and administered at a dose of 5×104 cells into both hind footpads of DO 11.10 TCR Tg mice. After 5 d, popliteal lymph nodes were harvested and lymph node cells were adjusted to 1×106 cells/mL in RPMI 10. The cultures (0.2 mL/well) were incubated in 96-well culture plates (Nunc, Roskilde, Denmark) in the presence of Dynabeads® Mouse T-Activator CD3/CD28 (Life Technologies, Oslo, Norway) at 37°C in a humidified atmosphere with 5% CO2. The culture supernatants were collected after incubation for 48 h, and the interferon (IFN)-γ and IL-4 concentrations were measured using enzyme-linked immunosorbent assay (ELISA) kits for quantification of murine IFN-γ and IL-4, respectively (R & D Systems, Minneapolis, MN, U.S.A.). The data were expressed as the mean (±standard deviation (S.D.)), and differences between means were analyzed using Student’s t-test with a two-tailed test of significance. Differences at p<0.05 were considered to be statistically significant.
RT-PCRIn order to determine the levels of mRNA expression for various cell surface molecules, mRNA was extracted from LDCs (1×105 cells) using a Dynabeads® mRNA DIRECT™ Micro Kit (Life Technologies, Oslo, Norway). The cDNA was then synthesized from the mRNA using a first-strand cDNA synthesis kit (GE Healthcare, Little Chalfont, Buckinghamshire, U.K.). PCR was performed using the following primers: β-actin (540 bp) 5′ primer, 5′-GTG GGC CGC TCT AGG CAC CAA-3′ and 3′ primer, 5′-CTC TTT GAT GTC ACG CAC GAT TTC-3′; CD40 (295 bp) 5′ primer, 5′-CCT GTA AGG AAG GAC AAC AC-3′ and 3′ primer, 5′-ATC ACG ACA GGA ATG ACC AG-3′; CD80 (312 bp) 5′ primer, 5′-GAA GAC CGA ATC TAC TGG CA-3′ and 3′ primer, 5′-GGA AGC AAA GCA GGT AAT CC-3′; CD86 (302 bp) 5′ primer, 5′-AGC CTG AGT GAG CTG GTA GT-3′ and 3′ primer, 5′-CCT GTT ACA TTC TGA GCC AG-3′; Delta 1 (318 bp) 5′ primer, 5′-TGC ACT GAC CCA ATC TGT CT-3′ and 3′ primer, 5′-CTC ACA GTT GGC ACC TGT AT-3′; Delta 3 (331 bp) 5′ primer, 5′-CTA CTG TGA AGA GCC TGA TG-3′ and 3′ primer, 5′-ACA GAC ATA GGC AGA GTC AG-3′; Delta 4 (307 bp) 5′ primer, 5′-TCA CCA GAC TGA GCT ACT CT-3′ and 3′ primer, 5′-ATG CTG CAG GTG CCA TGG AT-3′; Jagged 1 (314 bp) 5′ primer, 5′-ATC CGA GTG ACC TGT GAT GA-3′ and 3′ primer, 5′-TTG GTC TCA CAG AGG CAC TG-3′; Jagged 2 (300 bp) 5′ primer, 5′-GCT GTG ATG AGA ACT ACT AC-3′ and 3′ primer, 5′-TCT CAC AGT CAC AGT GCC AG-3′; T-cell immunoglobulin and mucin domain-containing protein (TIM)-4 (304 bp) 5′ primer, 5′-GTC CAG TTT GGT GAA GTG TC-3′ and 3′ primer, 5′-ACG TGG TCA CTG CTG TAC TG-3′. Each PCR was performed using a GeneAmp PCR System 9700 (Perkin-Elmer, Norwalk, CT, U.S.A.) in 25 µL of reaction mixture comprising 1.5 µL cDNA, 200 µM deoxynucleotide triphosphate mixture, 400 nM each PCR primer and 25 U/mL Ex Taq DNA polymerase (TaKaRa, Shiga, Japan). The reaction conditions were as follows: one 4-min cycle at 94°C, 35 cycles comprising 45 s at 94°C, 45 s at 61°C and 2 min at 72°C, followed by one 7-min cycle at 72°C, and the PCR products were separated on a 2% agarose gel containing ethidium bromide.
LDCs were pulsed with OVA peptide for 18 h in the absence or presence of tacrolimus or betamethasone. The LDCs were washed and injected into the hind footpads of DO 11.10 TCR Tg mice, and popliteal lymph node cells were harvested 5 d later. Subsequently, T-lymphocytes in the lymph node cells thus obtained were stimulated for 48 h through their surface CD3/CD28 molecules, and IFN-γ and IL-4 concentrations in the culture supernatants were then determined by ELISA. As shown in Fig. 1, LDCs treated with betamethasone, but not those treated with tacrolimus, inhibited the development of Th1 cells in a dose-dependent manner, as represented by suppressed IFN-γ production. Furthermore, betamethasone—but not tacrolimus—also suppressed Th2 cell development in lymph node cells by acting on LDCs, as verified by suppressed production of IL-4. However, because the concentrations of IL-17 (none; 742±107 pg/mL) and transforming growth factor (TGF)-β1 (none; 316±57 pg/mL) in the culture supernatants were not influenced by treatment with betamethasone (data not shown), inhibition of Th1 cell and Th2 cell development via betamethasone-treated LDCs would not involve Th17 cells and Treg cells.

LDCs were pulsed with OVA peptide for 18 h in the absence or presence of 1–10 µM tacrolimus or betamethasone. The OVA peptide-pulsed LDCs were then injected into the hind footpads of mice, and lymph node cells were harvested 5 d later. Lymph node cells were stimulated through their surface CD3/CD28 molecules, and the IFN-γ and IL-4 concentrations in the culture supernatants were determined by ELISA. Each culture was prepared in triplicate, and the mean value was obtained as a representative result for one experiment. The same experiment was repeated 6 times, and the results are expressed as the mean±S.D. (n=6). * p<0.05, ** p<0.01 versus non-treatment.
Subsequently, in order to clarify the mechanisms responsible for inhibition of Th1 cell and Th2 cell development via betamethasone-treated LDCs, LDCs were pulsed with OVA peptide for 18 h in the absence or presence of betamethasone or tacrolimus, and expression of mRNA for the cell surface molecules CD40, CD80, CD86, Delta 1, Delta 3, Delta 4, Jagged 1, Jagged 2 and TIM-4 was investigated using RT-PCR. As shown in Fig. 2, although tacrolimus had no influence on the mRNA expression of any cell surface molecules of LDCs, betamethasone suppressed the expression of CD40 and TIM-4 mRNA in LDCs.
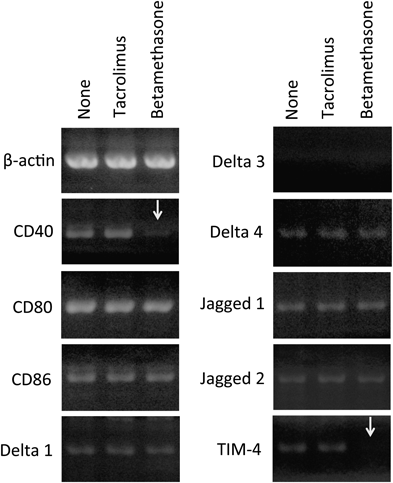
LDCs were pulsed with OVA peptide for 18 h in the absence or presence of 10 µM tacrolimus or betamethasone. Cytoplasmic mRNA was then extracted from the LDCs, reverse-transcribed and amplified by PCR using primer sets for β-actin, CD40, CD80, CD86, Delta 1, Delta 3, Delta 4, Jagged 1, Jagged 2, and TIM-4. The data shown are representative results of five independent experiments.
Th1/Th2 immune balance has a close relationship with various immunological diseases, including allergy. Many investigators have revealed that Th2 immunity is responsible for allergic immune responses and the subsequent pathogenesis of allergic inflammation diseases.8,9) AD is one of these allergy-related diseases: AD patients show a marked increase in the number of Th2 cells in both peripheral blood and acute skin lesions.1) Therefore, it has been proposed that Th2-type immune responses play a key pathogenetic role in AD, and this is supported by the presence of blood eosinophilia and enhanced serum immunoglobulin E (IgE) levels evident in most AD patients.10)
Immunosuppressive effects of betamethasone and tacrolimus involve inhibition of T cell function. The aim of this study was to clarify whether therapeutic effects of those drugs on AD were supported by inhibition of Th1 cell and/or Th2 cell development through LCs. In the present study, we observed that injection of betamethasone-treated LDCs induced suppression of both Th2 and Th1 cell development. This suppressive effect was also correlated with the decrease in the number of T cells proliferated in each culture (data not shown). Since betamethasone was washed out before LDCs-injection to mice, it would be reasonable to assume that betamethasone acted on LDCs and changed their function. To clarify the mechanism responsible for this Th1/Th2 down-regulation, expression of cell surface molecules on LDCs was confirmed by RT-PCR. The results of RT-PCR indicated that mRNAs for CD40, CD80, CD86, Delta 1, Delta 4, Jagged 1, Jagged 2 and TIM-4, but not for Delta 3, were expressed spontaneously in LDCs and predicted the presence of those cell surface molecules on LDCs. However, the expression of mRNA for CD40 and TIM-4 was suppressed in betamethasone-treated LDCs, but not in tacrolimus-treated LDCs. In mammals, four Notch receptors (Notch 1-4) and five Notch ligands (Delta1, Delta 3, Delta 4, Jagged 1 and Jagged 2) have been identified.11) Amsen et al.12) have presented evidence that different Notch ligands expressed on APCs are responsible for initiating Th1/Th2 differentiation in mice, and have concluded that, in APCs, Th1 adjuvant induces Th1 cell development through the expression of Delta members, whereas Th2 adjuvant induces Th2 cell development through the expression of Jagged members. Although down-regulation of Th1 and Th2 cell development by betamethasone was not related to the level of expression of Notch ligand mRNAs, expression of CD40 mRNA was suppressed by treatment with betamethasone. CD40 promotes production of IL-12 from APCs and induces Th1 differentiation.13) Furthermore, the expression of TIM-4 mRNA was suppressed by treatment with betamethasone. TIM-4 is expressed by DCs in lymphoid organs, and its ligand, TIM-1, is expressed by T cells. These molecules have been shown to be critical regulators of Th2 differentiation.14) In the present study, therefore, suppression of CD40 and TIM-4 expression would explain why LDCs treated with betamethasone were capable of suppressing both Th1 cell and Th2 cell development. In addition to the Th2-dominant cytokine response seen in the acute skin lesions of AD patients, chronic skin lesions are characterized by increased Th1 cytokine production with more or less stable Th2 cytokine production.15) Therefore, suppression of both Th1 and Th2 cell development would be a highly beneficial therapeutic strategy for patients with both acute and chronic AD inflammation.
The present findings indicate that betamethasone would be able to regulate Th1 cell and Th2 cell development through epidermal LCs, representing a new medicinal action of betamethasone. Our results suggest that topical application of betamethasone to acute lesional skin of AD patients would act on epidermal LCs and possibly inhibit the development of Th2 cells in vivo, thus being of benefit for the control of AD.
This work was supported by JSPS KAKENHI Grant Number 26460238.
The authors declare no conflict of interest.