2016 Volume 39 Issue 7 Pages 1150-1158
2016 Volume 39 Issue 7 Pages 1150-1158
Bone is a dynamic tissue that undergoes continuous remodeling coupled with the action of osteoblasts and osteoclasts. Osteoclast activity is elevated during osteoporosis and periodontitis resulting in excessive loss of trabecular and alveolar bone. Osteoclasts are formed in an inflammatory response to cytokine production receptor activator of nuclear factor-kappaB (NF-κB) ligand (RANKL) and bacterial challenge lipopolysaccharide (LPS). Carvacrol, a monoterpenic phenol present in Origanum vulgare and Thymus vulgaris, is a natural compound with known medicinal properties. We investigated the effects of carvacrol on osteoclast formation induced by RANKL and LPS. Carvacrol suppressed RANKL-induced formation of tartrate resistant acid phosphatase (TRAP)-positive multinucleated cells in RAW264.7 macrophages and human CD14+ monocytes. Furthermore, carvacrol inhibited LPS-induced osteoclast formation in RAW264.7 macrophages. Investigation of the underlying molecular mechanisms revealed that carvacrol downregulated RANKL-induced NF-κB activation in a dose-dependent manner. Furthermore, the suppression of NF-κB activation correlated with inhibition of inhibitor of kappaB (IκB) kinase (IKK) activation and attenuation of inhibitor of NF-κB (IκBa) degradation. Carvacrol potentiated apoptosis in mature osteoclasts by caspase-3 activation and DNA fragmentation. Moreover, carvacrol did not affect the viability of proliferating MC3T3-E1 osteoblast-like cells. Collectively, these results demonstrate that carvacrol mitigates osteoclastogenesis by impairing the NF-κB pathway and induction of apoptosis in mature osteoclasts.
Bone homeostasis is tightly coupled to the bone-forming activity of osteoblasts and bone-resorbing activity of osteoclasts.1) Osteoclasts are multi-nucleated cells that arise from hematopoietic progenitors of monocyte/macrophage lineage and specialize in bone resorption.2) Bone-loss related diseases such as osteoporosis and periodontitis are associated with increased osteoclast formation and elevated osteoclast activity.3–5)
Binding of receptor activator of nuclear factor-κB ligand (RANKL) to its receptor RANK in osteoclast precursors triggers trimerization of the receptor and subsequent activation of downstream signaling pathways that includes inhibitor of kappaB (IκB)-a/b kinase (IKK), nuclear factor kappa-B (NF-κB), mitogen-activated protein kinases (MAPKs)-p38, extracellular-signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK).6) Activation of these pathways leads to the formation of tartrate resistant acid phosphatase-positive (TRAP+) osteoclasts and bone resorption.
Development of apical periodontitis is associated with the presence of microorganisms in the root canal system.7) Gram-negative bacteria are the most common causative agents in endodontic infections.8) Bacterial death causes release of lipopolysaccharide (LPS) in the necrotic pulp and dental wall of periapically affected teeth.9) LPS elicits alveolar bone loss during periodontitis.10) LPS stimulates the secretion of cytokines by osteoclast precursors such as monocytes and macrophages, resulting in osteoclast formation and bone resorption in vitro and in vivo.11) Earlier studies have also shown that injection of LPS into mouse gingiva induces alveolar bone resorption.12)
Osteoclasts are the only cells present in the body possessing bone resorptive properties. Hence, either abrogating osteoclast formation or attenuating osteoclast activity is a fruitful approach to counteract bone loss. Natural products are a great source of lead compounds for developing novel drugs. Approximately 48.6% of currently approved U.S. Food and Drug Administration (FDA) drugs either originated or are derived from natural products.13) Accumulating lines of evidence suggest that several natural compounds may have potential anabolic effects on bone.14,15) Carvacrol (5-isopropyl-2-methylphenol), is a monoterpenic phenol found in oregano and thyme.16) Essential oils containing carvacrol have been widely used in various traditional medicines.17) Carvacrol possesses anti-inflammatory, antioxidant, and bactericidal properties.18) Topical application of carvacrol as a gel based formulation has been associated with the prevention of alveolar bone loss and growth of microorganisms in rats.19) However, the effects of carvacrol on osteoclastogenesis, and underlying molecular mechanisms have not been explored yet. This study analyzes the effects of carvacrol on osteoclast formation induced by RANKL and LPS, as well as its effects on RANKL signaling involved in osteoclastogenesis.
Dulbecco’s modified Eagle’s medium (DMEM), α-minimum essential medium (MEM) and heat-inactivated fetal bovine serum (FBS) were obtained from GIBCO (Grand Island, NY, U.S.A.) and Amersham (Little Chalfont, U.K.), respectively. Antibiotic-antimycotic solution containing 100 U/mL penicillin, 100 µg/mL streptomycin, and 0.25 µg/mL fungizone was supplied by Highveld Biological (Johannesburg, South Africa). Carvacrol, LPS from Escherichia coli O55:B5, phalloidin-Atto-488, and all other chemicals of research grade were obtained from Sigma-Aldrich Inc. (St. Louis, MO, U.S.A.). Human RANKL was supplied by Insight Biotechnology (Middlesex, U.K.). Mouse RANKL and human macrophage colony-stimulating factor (M-CSF) were acquired from R&D Systems (Minneapolis, MN, U.S.A.). All components for the magnetic separation of CD14+ monocytes were supplied by Miltenyi Biotec (San Diego, CA, U.S.A.). Alamar blue reagent, cell extraction buffer, NBT/BCIP Western Detection Chromogenic Kit were provided by Life Technologies (Carlsbad, CA, U.S.A.). Osteoassay surface multiwell plates were acquired from Corning Inc. (New York, NY, U.S.A.). The bicinchoninic acid (BCA) protein assay kit was purchased from Thermo Scientific (Rockford, IL, U.S.A.). Primary rabbit antibodies against IKKser176/180, IKK, IκBa, caspase-3 and poly[ADP-ribose]polymerase (PARP) were supplied by Cell Signaling Technology (Beverly, MA, U.S.A.). Primary rabbit antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was obtained from Abcam (Cambridge, MA, U.S.A.) and goat anti-rabbit alkaline phosphatase (ALP)-conjugated secondary antibody was procured from Life Technologies (Carlsbad, CA, U.S.A.). pNiFty2-secreted embryonic alkaline phosphatase (SEAP) plasmids and Zeocin were purchased from InvivoGen (San Diego, CA, U.S.A.). BioCellin™ transfection reagent was obtained from BioCellChallenge (Cedex, France).
Stock SolutionA 15 mg/mL stock solution of carvacrol was prepared in dimethyl sulfoxide (DMSO) (vehicle) and frozen as aliquots in −80°C until further use. Stock solutions were freshly diluted to working concentrations in complete culture medium before experiments. The final DMSO concentration in the culture medium did not exceed 0.1% (v/v).
Cell CultureRAW264.7 murine macrophages (#TIB-71) and MC3T3-E1 murine osteoblast-like cells (#2593) were purchased from American Type Culture Collection (ATC C, Rockville, MD, U.S.A.) and maintained in DMEM with 10% FBS. Cells were incubated at 37°C in a humidified atmosphere with 5% CO2.
Isolation of Human CD14+ Monocytes and Cell CultureAll the procedures and experimental protocols were approved by the Human Research Ethics Committee of the Faculty of Health Sciences, University of Pretoria (Protocol approval number: 67/2014) and in accordance with the 1964 Helsinki declaration and its later amendments. Eligible participants were asked to provide an additional written informed consent for enrolment. Human CD14+ monocytes were isolated from peripheral blood (40–60 mL) of healthy male donors (aged 18–35) as described previously using CD14+ magnetic beads as per manufacturer’s instructions (Miltenyi Biotec, San Diego, CA, U.S.A.). Cells were cultured in α-MEM supplemented with 10% FBS and incubated at 37°C in a humidified atmosphere with 7% CO2.
Alamar Blue AssayCD14+ monocytes (1×104/well) or RAW264.7 macrophages (5×103/well) were seeded in 96-well plates and allowed to adhere for 12 h, followed by exposure to increasing concentrations of carvacrol (3–15 µg/mL) for 48 h. Alamar blue assay was conducted as per manufacturer’s instructions (Life Technologies). Absorbance was measured at 570 nm with 600 nm as reference wavelength on a microplate reader (BioTek Instruments Inc., Winooski, VT, U.S.A.).
RANKL and LPS-Induced Osteoclast Differentiation and TRAP StainingRAW264.7 macrophages (in vitro model) and human CD14+ monocytes (ex vivo model) were used for osteoclast differentiation studies.
RANKL-treated RAW264.7 macrophages were differentiated into osteoclasts for 5 d as described previously.20) In brief, 5×103 cells per well were suspended in DMEM containing 10% FBS and seeded into sterile 96-well culture plates. Cells were stimulated with RANKL alone (15 ng/mL) or in combination with increasing concentrations of carvacrol (3–15 µg/mL). Cell culture media and factors were replaced every third day and differentiation was terminated on the fifth day unless otherwise stated.
CD14+ monocytes (4×104/well) were differentiated in the presence of M-CSF (25 ng/mL) and RANKL (30 ng/mL) for 14 d as described previously.21)
RAW264.7 macrophages (5×103/well) were exposed to 200 ng/mL of LPS in the presence or absence of carvacrol (15 µg/mL) and were differentiated for 7 d.
Osteoclast specific TRAP staining was performed using a leucocyte acid-phosphatase kit as per manufacturer’s directions (Sigma-Aldrich). TRAP+ cells with 3 or more nuclei were scored as osteoclasts. Photomicrographs were taken with a Zeiss Axiocam MRc5 camera attached to a Zeiss Axiovert 40 CFL microscope (Carl Zeiss AG, Oberkochen, Germany).
Resorption Pit Formation AssayThe bone resorption activity of RANKL-induced osteoclasts derived from RAW264.7 macrophages was assessed using osteoassay plates as per manufacturer’s instructions (Corning Inc.). Briefly, 2×104 cells per well were seeded onto 24-well osteoassay plates and exposed to RANKL (15 ng/mL) in the presence or absence of carvacrol (15 µg/mL) for 7 d. At the end of the culture, cells were washed off using a 5% bleach solution. Resorption pits were observed under a light microscope and quantified by ImageJ software.22)
Actin Ring Formation AssayActin rings of osteoclasts were detected by staining actin filaments with Atto-conjugated phalloidin as described elsewhere.23) Images were acquired using a fluorescence microscope (Carl Zeiss AG, Oberkochen, Germany).
Western Blot AnalysisCell lysates were prepared using cell extraction buffer (Life Technologies), supplemented with protease and phosphatase inhibitors (Sigma-Aldrich) and resolved on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. Purified proteins were quantified using a BCA protein assay kit as per manufacturer’s directions (Sigma-Aldrich). Proteins were electrotransferred to nitrocellulose membranes with Tris–glycine transfer buffer [25 mM Tris, 192 mM glycine, 20% methanol (v/v)], probed with each antibody and detected by NBT/BCIP substrate. Digital images of the blots were acquired using a flatbed scanner (Ricoh Aficio, Johannesburg, South Africa).
NF-κB SEAP AssaypNiFty2-SEAP (Invivogen) is an NF-κB-inducible reporter plasmid containing 5× NF-κB repeated transcription factor binding sites and a reporter gene—SEAP. RAW264.7 macrophages were stably transfected with GeneCellin™ transfection reagent. Stably transfected clones containing SEAP plasmid were selected with Zeocin. For promoter assay, transfected cell line was stimulated with 35 ng/mL of RANKL in the presence or absence of carvacrol (3–15 µg/mL) and SEAP assay was conducted after 48 h as per manufacturer’s protocol.
Mature-Osteoclast Survival AssayOsteoclasts were generated by RANKL treatment (15 ng/mL) from RAW264.7 macrophages for 5 d. Mature osteoclasts were exposed to carvacrol (15 µg/mL) for 24 and 48 h, respectively. At the end of the treatment, cells were stained for TRAP and images were acquired using a Zeiss Axiovert 40 CFL microscope (Carl Zeiss AG, Oberkochen, Germany). Multi-nucleated osteoclasts were counted.
Hoechst DNA Fragmentation AssayChanges in nuclear-DNA occurring due to apoptosis were observed by Hoechst staining using fluorescence microscopy. Mature osteoclasts derived from RANKL (15 ng/mL)-treated RAW264.7 macrophages after 4 d were exposed to carvacrol (15 µg/mL) for 24 h. Cells were incubated in Hoechst dye solution (5 µg/mL) for 5 min. Images were captured using a fluorescence microscope (Carl Zeiss AG, Oberkochen, Germany). Percentage of apoptotic cells were calculated by tracking the number of cells with apoptotic nuclear condensation using ImageJ software.
Lactate Dehydrogenase (LDH) AssayMature osteoclasts derived from RAW264.7 macrophages were treated with carvacrol for 24 and 48 h respectively. LDH activity from culture supernatants were analyzed as described earlier.24) Briefly, cells (5×103/well) seeded in 96-well plates were differentiated for 4 d in the presence of RANKL (15 ng/mL) and further incubated in combination with carvacrol (3–15 µg/mL) for 24 and 48 h. At each time-point culture supernatants were collected and reconstituted with LDH assay buffer and were further incubated for 30 min. Absorbance was measured at 490 nm on a microplate reader (BioTek Instruments Inc., Winooski, VT, U.S.A.).
Statistical AnalysisData are representative of three independent experiments unless otherwise stated and are represented as the mean±standard deviation (S.D.). Statistical analysis was performed by t-test for comparison between two groups or one-way ANOVA followed by Bonferroni post hoc test when more than two groups were compared using Graph Pad Prism Software (GraphPad Software Inc., CA, U.S.A.). p<0.05 was regarded as statistically significant.
RAW264.7 macrophages and CD14+ monocytes were treated with 3–15 µg/mL of carvacrol (Fig. 1A) to analyze cell viability by alamar blue assay. At tested concentrations, carvacrol did not exert cytotoxic effects on the studied cell lines (Figs. 1B, C). Hence, these concentrations were chosen to study the effects of carvacrol in downstream experiments.

A) Molecular structure of carvacrol. B) CD14+ monocytes (1×104/well with 25 ng/mL M-CSF) and C) RAW264.7 macrophages (5×103/well). Cells were treated with indicated concentrations of carvacrol for 48 h and cell viability of proliferating cells was measured using alamar blue assay. D) RAW264.7 macrophages (5×103/well) were differentiated with RANKL (15 ng/mL) alone or in combination with carvacrol at indicated concentrations for 5 d. Osteoclasts stain purple/pink in the presence of TRAP (scale bars: 100 µm). E) TRAP+ cells containing three or more nuclei were counted as osteoclasts. Black bars represent RANKL-treated cells whereas white bars illustrate RANKL and carvacrol co-treated cells. F) CD14+ monocytes (4×104/well) were differentiated with RANKL (30 ng/mL) and M-CSF (25 ng/mL) or in combination with carvacrol at indicated concentrations for 14 d (scale bars: 100 µm). G) The number of TRAP+ osteoclasts formed were counted and represented in a graphical format. Black bars represent RANKL-treated cells whereas white bars illustrate RANKL and carvacrol co-treated cells. The results are the mean±S.D. percent of control and are representative of three independent experiments performed in triplicate (*** p<0.0001 versus RANKL). Black arrows indicate TRAP+ osteoclasts.
RANKL is the key cytokine and stimulator of osteoclastogenesis. Hence, we studied the effects of carvacrol on osteoclast differentiation induced by RANKL. RAW264.7 osteoclast progenitor cells were treated with RANKL (15 ng/mL) in the presence or absence of various concentrations (3–15 µg/mL) of carvacrol and the cells were allowed to differentiate into osteoclasts for 5 d. We found that RANKL-treated cells differentiated into TRAP+ giant multinucleated osteoclasts (Fig. 1D). On the contrary, carvacrol significantly decreased RANKL-induced differentiation of cells into osteoclasts (Figs. 1D, E).
To further examine whether carvacrol could also modulate differentiation of human CD14+ monocytes ex vivo, we exposed these cells to carvacrol. Results indicated that RANKL robustly induced osteoclast formation, whereas carvacrol significantly inhibited this differentiation in CD14+ monocytes (Figs. 1F, G).
Carvacrol Inhibits LPS-Induced Osteoclast FormationBacterial LPS is an inflammatory modulator that is involved in alveolar bone loss and osteoclastogenesis during periodontitis. To elucidate whether inhibitory effects of carvacrol on osteoclastogenesis could also be interpolated on LPS-induced osteoclast formation, we studied osteoclast differentiation in the presence of LPS. Treatment of RAW264.7 macrophages with LPS (200 ng/mL) resulted in the formation of multi-nucleated TRAP+ osteoclasts, whereas co-treatment with carvacrol (15 µg/mL) significantly reduced LPS-induced osteoclastogenesis (Figs. 2A, B).
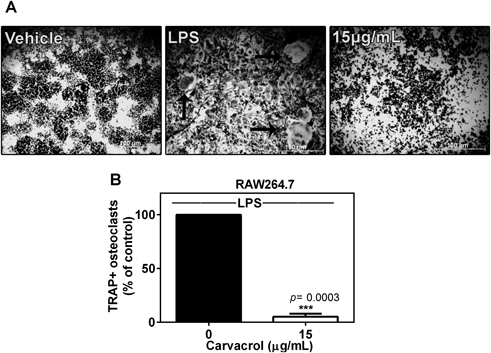
A) RAW264.7 macrophages (5×103/well) were differentiated for 7 d in the presence of LPS (200 ng/mL) alone or in the co-presence of carvacrol (15 µg/mL) (scale bars: 100 µm). B) Resultant TRAP+ osteoclasts containing three or more nuclei were counted. Data represent the mean±S.D. percent of control and are representative of three independent experiments performed in triplicate (*** p=0.0003 versus LPS). Black arrows indicate TRAP+ osteoclasts. Black bars represent RANKL-treated cells whereas white bars illustrate RANKL and carvacrol co-treated cells.
During differentiation osteoclast progenitors fuse into multinucleated osteoclasts and form large actin rings which aid osteoclasts in attachment to the bone and resorb the matrix. As can be seen in Fig. 3A, RANKL (15 ng/mL) induced the fusion of RAW264.7 macrophages into osteoclasts resulting in formation of actin rings visible as green fluorescence. Conversely, and in concordance with the osteoclastogenesis results, RAW264.7 macrophages co-stimulated with RANKL and carvacrol (15 µg/mL) failed to fuse into osteoclasts and develop actin rings (Fig. 3A). Furthermore, owing to reduced osteoclastogenesis and actin ring formation, resorption pit formation activity was attenuated in RANKL and carvacrol (15 µg/mL) co-treated cells (Fig. 3B). However, RANKL treated cells clearly demonstrated bone resorptive activity (Fig. 3B).

RAW264.7 macrophages (5×103/well) were differentiated into osteoclasts in the presence of RANKL (15 ng/mL) alone or in the co-presence of carvacrol (15 µg/mL), and A) were stained for actin ring formation with phalloidin (scale bars: 20 µm) or B) were tested for resorption pit formation on osteoassay plates (scale bars: 50 µm). Resorption percentages quantified with ImageJ are indicated in the figure. Light areas are the resorbed areas.
NF-κB pathway plays an indispensable role in osteoclast differentiation. To elucidate the effects of carvacrol on RANKL-induced NF-κB activation in RAW264.7 macrophages, NF-κB-secreted-alkaline phosphatase-promoter reporter assay (SEAP) was conducted. RAW264.7 macrophages were stably transfected with pNiFty2-SEAP plasmid containing 5× NF-κB repeated transcription factor binding sites as described in methods. Cells were either treated with RANKL alone (35 ng/mL) or in combination with carvacrol (3–15 µg/mL) for 48 h. As seen in Fig. 4A, carvacrol potently suppressed RANKL-induced NF-κB promoter activity at all the tested concentrations.

A) RAW264.7 macrophages stably transfected with NF-κB-pNiFty2-SEAP plasmids were either treated with RANKL (35 ng/mL) alone or in combination with indicated concentrations of carvacrol. SEAP activity from culture supernatants were measured after 48 h. Data shown is SEAP activity ±S.D. percent of control. Results are representative of three independent experiments performed in triplicate (*** p<0.0001 versus RANKL). Black bars represent RANKL-treated cells whereas white bars illustrate RANKL and carvacrol co-treated cells. RAW264.7 macrophages were pretreated with or without carvacrol (15 µg/mL) for 4h prior to RANKL (35 ng/mL) stimulation at the indicated time points. Cell lysates were analyzed by Western blot with B) phosphorylated and total-IKK, C) IκBa and GAPDH antibodies respectively.
RANKL-induced osteoclastogenesis involves phosphorylation and activation of IKK resulting in activation of downstream signaling events. Hence, we next analyzed whether carvacrol affects the activation or expression of IKK. Treatment of RAW264.7 macrophages with RANKL (35 ng/mL) led to a sharp increase in phosphorylated IKK levels in a time-dependent manner. In contrast, co-treatment with carvacrol (15 µg/mL) alleviated RANKL-induced IKK phosphorylation (Fig. 4B). Furthermore, total-protein expression levels of IKK remained unaffected by carvacrol treatment (Fig. 4B).
Carvacrol Inhibits RANKL-Induced IκBa DegradationDegradation of the inhibitory protein IκBa is a prerequisite step in NF-κB activation. Hence, to determine whether inhibition of IKK activation also led to abrogation of IκBa degradation, we analyzed its protein levels by Western blot. RANKL-treatment (35 ng/mL) reduced IκBa protein levels within 15 min of exposure and the expression returned to initial levels after 60 min. On the other hand, co-treatment with carvacrol (15 µg/mL) perturbed RANKL-induced IκBa degradation (Fig. 4C). At these conditions carvacrol had no effect on MAPK pathways (results not shown).
Carvacrol Negatively Affects Mature Osteoclast NumbersSince carvacrol inhibited osteoclastogenesis at an early stage of differentiation, we sought to find out whether carvacrol has an inhibitory effect on the survival of mature osteoclasts. To examine whether carvacrol induces apoptosis in mature osteoclasts, the multinucleated cells were treated with carvacrol for different time periods. RANKL-induced (15 ng/mL) TRAP+ mature osteoclasts were exposed to carvacrol (15 µg/mL) in the presence of RANKL for 24 and 48 h. Carvacrol reduced the mature osteoclast numbers after 24 h and this effect was more prominent after 48 h. However, cells treated with RANKL alone remained unaffected (Figs. 5A, B).

A) RAW264.7 macrophages (5×103 cells/well) were treated with RANKL (15 ng/mL) and differentiated for 5 d. Cells were either exposed to carvacrol (15 µg/mL) alone; RANKL alone (15 ng/mL) or were co-treated with both the factors for 24 and 48 h (scale bars: 50 µm). B) Multi-nucleated osteoclasts were counted and plotted as percent of control in graphical format. Data are the mean±S.D. percent of control and are representative of three independent experiments performed in triplicate (*** p=0.0002 versus RANKL (24 h); p<0.0001 versus RANKL (48 h). Black bars represent RANKL-treated cells whereas white bars illustrate RANKL and carvacrol co-treated cells.
To further elucidate the mechanisms of action of carvacrol on mature osteoclast survival we conducted LDH assay for necrosis and Hoechst-DNA fragmentation assay for studying apoptosis. Exposure of mature osteoclasts to carvacrol (3–15 µg/mL) in combination with RANKL (15 ng/mL) did not result in significant LDH release compared to control osteoclasts (Fig. 6A). On the other hand, mature osteoclasts treated with carvacrol (15 µg/mL) in the presence of RANKL demonstrated nuclear fragmentation and condensation, a typical hallmark of apoptosis (Figs. 6B, C). Furthermore, in these conditions carvacrol alone did not induce nuclear fragmentation in mature osteoclasts.

A) RAW264.7 macrophages (5×103 cells/well) were treated with RANKL (15 ng/mL), differentiated for 4 d and then incubated with RANKL alone or in combination with carvacrol (3–15 µg/mL) for 24 and 48 h. Necrosis was analysed by LDH assay. B) Osteoclasts derived from RAW264.7 macrophages were incubated with carvacrol (15 µg/mL) in the co-presence of RANKL (15 ng/mL) for 24 h. Apoptosis indicated by nuclear fragmentation was assessed by Hoechst assay (scale bars: 20 µm). C) Number of cells with fragmented DNA in three different fields in three experiments were counted and plotted in a graph format. Data indicates the mean±S.D. percent of control. Results are representative of three independent experiments performed in triplicate (*** p<0.0001 versus RANKL, RC=RANKL+carvacrol). D) Carvacrol activates caspase-3 in mature osteoclasts. RAW264.7 macrophages were differentiated in the presence of RANKL (15 ng/mL) for 4 d and then incubated in combination with carvacrol (15 µg/mL) for 24 and 48 h. Cell lysates were analyzed by Western blot analysis (R=RANKL, R+C=RANKL+carvacrol).
To delineate the molecular mechanisms involved in carvacrol induced apoptosis we analyzed caspase-3 and PARP activation in carvacrol-treated mature osteoclasts after 24 and 48 h. Mature osteoclasts exposed to carvacrol (15 µg/mL) along with RANKL (15 ng/mL) showed a remarkable increase in cleaved caspase-3 active form (Fig. 6D). Moreover, carvacrol stimulation caused cleavage of PARP, an intracellular substrate of caspase-3 (Fig. 6D).
Effects of Carvacrol on the Viability of Proliferating MC3T3-E1 Osteoblast-Like CellsThe action of osteoblasts and osteoclasts are closely coupled to each other. To find out whether carvacrol is cytotoxic to murine MC3T3-E1 osteoblast-like cells, we further conducted alamar blue cell viability assay. Treatment of MC3T3-E1 cells with carvacrol (3–15 µg/mL) did not affect the viability of these cells under proliferation conditions (Fig. 7).

Cells were treated with the indicated concentrations of carvacrol for 48 h, cell viability was measured by alamar blue assay. Data are expressed as the mean±S.D. percent of control and are representative of three independent experiments performed in triplicate.
Bone remodeling occurs throughout an individual’s lifetime.1) Although this process is tightly regulated, during certain pathological conditions bone resorption overtakes bone formation causing an imbalance.25) Diseases such as osteoporosis and periodontitis induce a paramount increase in osteoclast formation and activity.5) Osteoclasts, the only cells in the body involved in bone resorption are a crucial target for alleviating bone loss. In this study, we investigated the effects of carvacrol on aberrant osteoclastogenesis induced by various pathological stimuli such as RANKL and bacterial-LPS. The results demonstrate that carvacrol potently inhibited osteoclast formation by suppressing the NF-κB pathway and negatively affected the survival of mature osteoclasts by inducing apoptosis in these cells.
Osteoclasts are terminally differentiated multi-nucleated cells originating from the hematopoietic progenitors of monocyte/macrophage lineage.26) In healthy individuals, the process of osteoclastogenesis and bone resorption is maintained at subtle levels to allow a regulated rate of bone remodeling.1) However, various pathophysiological stimuli or aberrant signaling can cause an unregulated increase in bone loss.5) RANKL is a cytokine that regulates osteoclast formation.6) During diseases such as osteoporosis, rheumatoid arthritis and cancer-induced osteolysis this signaling pathway is disturbed leading to a vicious cycle of bone loss.5) Similarly, LPS can trigger inflammation resulting in bone loss during diseases such as osteomyelitis and periodontitis.10) LPS from E. coli has been reported to be a potent inducer of inflammatory cytokine secretion and osteoclast formation.11,27,28) LPS from E. coli is a good model to study bacteria-induced osteoclastogenesis. Natural products are a great source of affordable and efficacious compounds with potent pharmacological properties and have led to development of many drugs.13) In search of novel anti-osteoclastogenic compounds, we evaluated the efficacy of carvacrol on osteoclast formation. We found that carvacrol potently mitigated RANKL and LPS-induced TRAP+ osteoclast formation without exerting cytotoxic effects on proliferating MC3T3-E1 osteoblast-like cells. Cellular events during osteoclast formation includes fusion of progenitor cells and cytoskeleton rearrangement resulting in actin ring formation.29) These rings aid osteoclasts in attachment, sealing zone formation and resorption of bone. We found that owing to perturbed osteoclastogenesis, carvacrol-treated osteoclast progenitor cells failed to form actin rings resulting in inhibition of resorption pit formation.
The NF-κB pathway plays an important role in osteoclastogenesis. NF-κB knockout mice display severe osteopetrosis due to complete lack of osteoclasts.30) During osteoclastogenesis, the NF-κB pathway and MAPK pathways (p38, JNK and ERK) are activated leading to downstream signaling events and target gene expression.6) It has been reported that RANKL signaling and LPS can activate the NF-κB pathway in osteoclast progenitors triggering osteoclast formation.6,31,32) Hence, suppression of NF-κB activation is an effective approach to reduce bone loss. Our results demonstrate that NF-κB was activated by RANKL via phosphorylation of IKK and subsequent degradation of IκBa. Furthermore, we found that carvacrol inhibited NF-κB activation by mitigating IKK and IκBa activation.
Inhibition of osteoclast formation is one of the strategies to reduce bone loss. However, certain anti-osteoporotic drugs have the dual potential of inducing apoptosis in mature osteoclasts suggesting an important alternative strategy to treat bone-loss diseases with excessive osteoclastogenesis/activity.33) Additionally, selective targeting of mature osteoclasts makes an interesting and novel strategy for osteoporosis treatment. Hence, we further investigated apoptosis inducing potential of carvacrol in mature osteoclasts. Intriguingly, carvacrol reduced the numbers of mature osteoclasts in a time-dependent manner, suggesting carvacrol negatively regulates survival of mature osteoclasts. Hallmarks of apoptosis include nuclear condensation, chromatin fragmentation and caspase activation.34) We found that carvacrol induced-apoptosis correlated with nuclear DNA fragmentation, a characteristic feature of apoptosis. LDH is a soluble cytoplasmic enzyme that is present in almost all cells and is released into extracellular space when the plasma membrane is damaged during necrosis.24) We found that carvacrol treatment did not trigger necrotic cell death as seen through insignificant amounts of LDH release in treated and non-treated cells. Caspase-3 is the critical mediator of apoptosis. Activation of caspase-3 leads to the activation of apoptotic pathway which further involves cleavage and activation of its substrate PARP.35) Treatment of mature osteoclasts with carvacrol led to cleavage of caspase-3 and PARP in a time dependent manner. These results indicate that carvacrol induces apoptosis in mature osteoclasts through caspase-3 pathway. Earlier reports have suggested that NF-κB pathway plays an important role in osteoclast survival.36) Inhibition of NF-κB triggers caspase-3 cleavage and activation of apoptotic pathway in osteoclasts.36) Since carvacrol effectively inhibited the NF-κB pathway, it is possible that this mechanism contributed to the apoptotic activity of carvacrol.
In conclusion, results obtained in this study indicate that carvacrol can potently inhibit osteoclastogenesis induced by RANKL and bacterial-LPS. In addition to its anti-osteoclastogenic potential, carvacrol simultaneously induced apoptosis in mature osteoclasts at least in part by activation of caspase-3. These results suggest that carvacrol has inhibitory effects on osteoclasts and could be a potential compound for treating osteoporosis and periodontitis.
This study was supported by Grants from RESCOM, University of Pretoria; the University of Pretoria Vice Chancellor’s Postdoctoral Research Fellowship; the University of Pretoria’s Strategic Institutional Research Theme in Food, Nutrition and Well-being; and in part by the Struwig-Germeshuysen Cancer Research Trust, South Africa.
The authors declare no conflict of interest.