2016 年 39 巻 7 号 p. 1201-1205
2016 年 39 巻 7 号 p. 1201-1205
Nanomaterials (NMs) are defined as those which have nanostructured components less than 100 nm in diameter. They are widely used in various fields such as medicine, cosmetics, and the food industry. However, the toxicological effects of NMs are less well understood than their applications. In particular, the skin is exposed to the environment at all times, so is easily influenced by NMs. In this study, we investigated the skin permeability and toxicological properties of well-dispersed amorphous silica particles with diameters ranging from 70 to 1000 nm, to aid in the safe application of NMs. Amorphous silica particles of 70 nm in size (nSP70) penetrated the living epidermis, following pretreatment with acetone/diethyl ether to improve skin permeation. The application of unmodified nSP70, carboxyl group-modified nSP70, or amino group-modified nSP70 for long durations caused little skin irritation at the application site. Under the present experimental conditions, few adverse systemic effects were evident from blood tests and histopathologic examination. These results suggest that decreasing particle size increases the NMs skin permeability, but remarkably little corresponding skin irritation is observed.
Many nanomaterials (NMs) with controlled particle sizes of below 100 nm have been developed. The small size of NMs provides them with useful physicochemical properties and functionality that differ from those of bulk materials. NMs such as amorphous nano-sized silica particles (nSPs) or titanium dioxide nanoparticles are widely used in consumer and industrial applications.1–4)
NMs also exert biologic effects that are not caused by conventional submicron- and micro-sized particles. NMs may therefore induce adverse effects, owing to their small size. In several biological systems, NMs have been shown to interact in ways differing to that of their corresponding bulk materials.5,6) Several reports have suggested that exposure of cells to nSPs (as models NMs) could induce cytotoxicity and inflammation, and that intravenous administration of nSPs could cause acute liver inflammation and progression to death.7–11) In addition, some reports suggest that the size and surface properties of NMs are important for inducing pharmacologic or pharmacokinetic alterations and cellular responses.12–15) These suggest that NMs can cause unexpected adverse reactions when they invade the body. Therefore, basic information about the characteristics of NMs and their in vivo and in vitro application is essential for the development of safe NMs.
The skin is a major entry point for external substances because of its large surface area and environmental exposure. The skin has a high frequency of exposure to NMs because NMs such as nSP-containing cosmetics or sunscreens are often applied daily. It is generally thought that particles and macromolecules such as peptides and proteins cannot penetrate into the skin after topical application because the stratum corneum acts as a physical barrier.16,17) Therefore, these submicron particles appear to have little adverse effect on the skin. However, recent studies suggest that some NMs can penetrate the stratum corneum into skin because of their differing physicochemical properties.18–20) If they penetrate the stratum corneum into skin and enter the blood, NMs might cause skin inflammation or tissue injury. Therefore, to develop safe NMs, it is worthwhile considering the possibility of skin permeation and toxicological effects by NMs.
In this study, to assess skin penetration and potential toxicological effects of NMs to the body after dermal exposure both in vitro and in vivo, we first confirmed the skin penetration of well-dispersed nSPs as model NMs, with diameters ranging from 70 to 1000 nm. Then, we analyzed in vivo toxicity of several nSPs over long application periods. nSPs are suitable as model NMs for investigating the influence of NMs on the skin because they are well dispersed, easily-handled, and widely used in cosmetics.10)
Fluorescent isothiocyanate-labeled nSPs with diameters of 70 nm (designated F-nSP70), conventional submicron or microsilica particles with diameters of 300 nm and 1000 nm (designated F-nSP300 and F-mSP1000, respectively), unmodified nSP70 (designated nSP70) carboxyl group-modified nSP70 (designated nSP70-C), and amino group-modified nSP70 (designated nSP-70-N) suspended in distilled water were purchased from Micromod Partikeltechnologies (Rostock/Warnemuende, Germany). Each silica particle suspension was sonicated for 5 min and then vortexed for 1 min immediately prior to use. The fluorescein intensity of each nSP was almost the same.
AnimalsICR mice (female, 6 weeks) were purchased from Japan SLC, Inc. All animals were specific pathogen-free and maintained in the experimental animal facility at the Osaka University. The experiments were conducted in accordance with the guidelines provided by the Animal Care and Use Committee of Osaka University.
In Vitro Skin Penetration Assay Using TESTSKIN LSE-HighTESTSKIN LSE-high is an artificial skin model containing no pores, and was obtained from Toyobo (Osaka, Japan). The dermal portion of TESTSKIN LSE-high was placed on a polycarbonate membrane in contact with 1.2 mL assay medium in a 6-well plate. A polyethylene ring was affixed to the surface of the TESTSKIN LSE-high with silicone sealant, and F-nSP70, F-nSP300, or F-mSP1000 was applied to the TESTSKIN LSE-high at 2.5 mg/100 µL with or without isopropyl myristate (final concentration, 5%w/w). After 3 or 9 h of incubation, aliquots from the receptor compartment were withdrawn, and the penetration levels were analyzed by measuring the fluorescence intensity of the silica particles. The amount of each silica particle was normalized with the measured fluorescence intensity.
Application Procedure of Each Silica ParticleF-nSP70, F-nSP300, or F-mSP1000 (each 125 µg/5 µL) was applied to the auricular skin of ICR mice for 3 consecutive days for the skin penetration assay or for 28 consecutive days in the toxicity assessment. The skin was pretreated with mixed acetone–diethyl ether (1 : 1) solution before application to destroy the barrier function of stratum corneum.21) For 12 h after application, a device on the animal collar was used to prevent mice from licking auricle skins.
In Vivo Skin Penetration Assay Using ICR MiceTwenty-four hours after the last application, auricle skins were harvested and epidermal sheets were prepared according to the method of Mackenzie and Squier.22) Epidermal sheets were fixed in cold acetone and mounted by Prolong Gold antifade reagent with 4′,6-diamidino-2-phenylindole. Confocal laser scanning microscopy (TCS-SP2; Leica Microsystems GmbH, Wetzlar, Germany) was used to photograph of the epidermal sheets.
In Vivo Toxicity Evaluation of Each Type of nSP70Twenty-four hours after the last application, the test sites were observed and scored for signs of erythema or edema according to the Draize dermal scoring criteria.23) This scale was used to assess irritation as follows: 0, no erythema or edema; 1, very slight erythema and/or barely perceptible edema; 2, well-defined erythema and/or slight edema; 3, moderate to severe erythema or moderate edema; and 4, severe erythema and/or edema. Serum, skin, liver, kidney, spleen, lung, brain, and lymph node were also collected from each mouse. Each tissue (skin, liver, kidney, spleen, lung, brain, and lymph node) was fixed in 10% neutral-buffered formalin at pH 7.4 (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and embedded in paraffin. Sections (5 µm) were prepared for hematoxylin and eosin staining. Histopathologic examinations were performed at the Applied Medical Research Laboratory (Osaka, Japan). Inflammation level was scored according to the severity of the inflammatory cell infiltration: −, no; ±, rare; +, mild; ++, moderate; +++, severe. To avoid bias, scoring was performed by an investigator without knowledge of the sample treatment conditions. In addition, a commercial kit was used for the quantitative colorimetric determination of glutamate oxaloacetate transaminase (GOT), glutamic pyruvic transaminase (GPT) (Transaminase-CII-Test-Wako, Wako Pure Chemical Industries, Ltd.), and creatinine (CRE) (Creatinine-test-Wako, Wako Pure Chemical Industries, Ltd.) from mice serum.
First, we evaluated the in vitro penetration of F-nSP70, F-nSP300, and F-mSP1000 through the stratum corneum of tissue-engineered skin (TESTSKIN LSE-high). Isopropyl myristate was used as additive because it is used in cosmetics to promote penetration. As shown in Fig. 1, after 3 or 9 h of incubation, a small percentage of silica particles applied on the surface of TESTSKIN LSE-high penetrated the epidermis with or without the influence of isopropyl myristate and were detected in the donor chamber, although measurements of the amount of penetrated F-nSP70 varied widely. On the other hand, F-nSP300 and F-mSP1000 did not penetrate the TESTSKIN LSE-high, even with the assistance of isopropyl myristate (Fig. 1). One pathway for substance diffusion through the skin is via skin appendages such as hair follicles and glands.24) nSP70 penetrated TESTSKIN-LSE-high, which is a skin model without appendages. Therefore skin appendages need not necessarily contribute to the penetration of nSP70 through skin. It was reported that skin penetration of substances can also occur via paracellular routes or transcellular routes.24,25) Further investigation about skin penetration is required in the future.
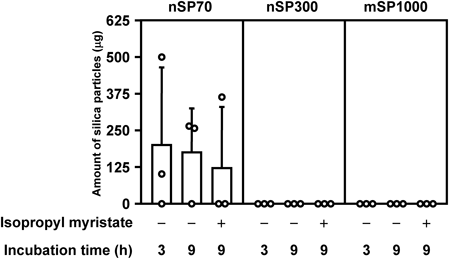
F-nSP70, F-nSP300, or F-mSP1000 (each 2.5 mg/100 µL) was applied to TESTSKIN LSE-high in the donor chamber with or without isopropyl myristate (final concentration, 5%w/w) and then incubated for 3 or 9 h at 37°C. Penetration levels were analyzed by measuring the fluorescence intensity of each fluorescence-labeled silica particle that penetrated the TESTSKIN LSE-high to the receptor chamber. The amount of each silica particle penetrating TESTSKIN LSE-high was normalized with the measured fluorescence intensity. Mean values are presented in the bar graph. Circles represent individual data points.
We then examined the skin penetration of F-nSP70, F-nSP300, and F-mSP1000 in vivo, by observing epidermal sheets by confocal laser scanning microscopy after topical application. Epidermal sheets consist of the stratum corneum and living epidermis, which consist of dead cells without nuclei and live cells such as keratinocyte and Langerhans cells, respectively. Thus, it is easy to distinguish between them by nuclear staining. If fluorescence derived from silica particles is detected in the epidermal layer with nuclear staining, then the particles have penetrated the stratum corneum into the skin. Silica particles were applied on acetone/diethyl ether-treated skin for 3 d to evaluate the skin penetration in situations of high skin permeability. As shown in Fig. 2, subsequent observation of the epidermal sheets with nuclear staining revealed fluorescence derived from F-nSP70, but not from F-nSP300 and F-mSP1000. This suggested that F-nSP70 penetrates the stratum corneum into the living epidermis in vivo. No fluorescence from F-nSP70 was observed in the living epidermal sheets of the skin, without pretreatment using acetone/diethyl ether (data not shown). These results suggested that F-nSP70 invades the skin under the stratum corneum in situations of high skin permeability, such as when combined with a penetration enhancer. Other groups have reported that nSPs topically applied on the skin penetrated the stratum corneum and were captured by Langerhans cells as antigen-presenting cells in the epidermis.10) This finding supported our results that decreasing nSP particle size will promote penetration through the stratum corneum. In our current in vivo experiment, no information was revealed about the amount of F-nSP penetrating the stratum corneum or the depth to which it penetrated, so further quantitative analysis is required. Additionally, further investigation should focus on the relationship between the dosed topical nSP70 and penetrated nSP70, and the maximum particle size that can penetrate the stratum corneum. Taken together, these in vitro and in vivo data suggest that smaller NMs have the potential to penetrate the skin in situations of high skin permeability. The mechanism of skin penetration by nSP70 will be examined in the future.
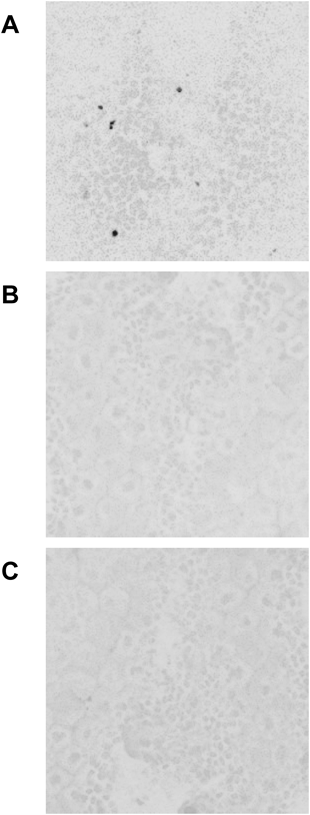
ICR mice were applied with F-nSP70 (A), F-nSP300 (B), or F-mSP1000 (C) (each 125 µg/5 µL) to auricle skin, which was pretreated with mixed acetone/diethyl ether solution, for 3 d. Twenty-four hours after the last application, auricles were harvested and epidermal sheets were prepared. The epidermal sheets were observed under a confocal laser microscope. The colors in the images were inverted for improving the visualization in black and white. Black; silica particle, gray; DAPI.
The above in vitro and in vivo penetration analysis suggested that decreasing particle size increased skin permeability. After penetration, nSP70 may migrate into the blood and spread systemically, potentially circulating around the body. Previous reports demonstrated that the intravenous administration of nSP70 caused hepatic damage or function disorder, in the worst cases leading to death.7–11)
We investigated local irritation on the skin and systemic adverse reactions in mice after topically applying nSP70 over a long period, at the same dosage used in the above skin penetration analysis. Several reports have demonstrated that the characteristics of NMs, such as surface properties and size, are important factors for the induction of pharmacologic or pharmacokinetic alterations and cellular responses.12–15) Three types of nSP70 were used in this experiment: unmodified nSP70, carboxyl group-modified nSP70 (nSP70-C), and amino group-modified nSP70 (nSP-70-N).
To assess local toxicity, we evaluated erythema and edema at the application site based on Draize scoring after a long application period for each type of silica particle to the non-treated intact skins and the acetone/diethyl ether-treated skins. No edema was observed in any mice (data not shown). In one of five mice with the intact skin, the application of nSP70 induced slight erythema that was rapidly resolved (Table 1). The rapid disappearance of the erythema suggested that the degree of skin irritation caused by the application of nSP70 was very mild and temporary. Other application groups showed no erythema. No inflammation at the application site was detected in any of the mice (Table 2). The local toxicity tests indicated that the application of nSP70 with or without surface modification caused little irritation or inflammation to the skin with or without the influence of the acetone/diethyl ether-treatment.
| Draize score | Intact skin | Acetone/diethyl ether-treated skin | ||||||
|---|---|---|---|---|---|---|---|---|
| nSP70 | nSP70-N | nSP70-C | — | nSP70 | nSP70-N | nSP70-C | — | |
| 0 | 4/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 |
| 1 | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| 2 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| 3 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| 4 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
nSP70, nSP70-C, or nSP-70-N (each 125 µg/5 µL) was applied to skin with or without pretreatment of mixed acetone/diethyl ether solution for 28 d. Twenty-four hours after the last application, the application site was observed. The degree of erythema on the skin of the mice was evaluated according to Draize scoring: 0, no erythema; 1, very slight erythema; 2, well-defined erythema; 3, moderate to severe erythema; and 4, severe erythema.
| Intact skin | Acetone/diethyl ether-treated skin | |||||||
|---|---|---|---|---|---|---|---|---|
| nSP70 | nSP70-N | nSP70-C | — | nSP70 | nSP70-N | nSP70-C | — | |
| Skin | − | − | − | − | − | − | − | − |
| Liver | − | − | − | − | − | − | − | − |
| Kidney | ± | ± | ± | ± | ± | ± | ± | ± |
| Spleen | + | + | + | + | + | + | + | + |
| Lung | − | − | − | − | − | − | − | − |
| Brain | ± | ± | ± | ± | ± | ± | ± | ± |
nSP70, nSP70-C, or nSP-70-N (each 125 µg/5 µL) was applied to skin with or without pretreatment of mixed acetone/diethyl ether solution for 28 d. Twenty-four hours after the last application, skin, liver, kidney, spleen, lung, and brain were harvested. Histopathologic scores of liver, kidney, spleen, lung, and brain harvested from mice with application of each silica particle were assigned based on hematoxylin and eosin-stained sections. Histopathologic changes were expressed as follows: −, no; ±, rare; +, mild; ++, moderate; +++, severe.
We then investigated systemic side effects caused by application of each type of silica particle. The histopathologic scores for liver, kidney, spleen, lung, and brain after applying each type of silica particle were the same as those for the non-treated control group, and none of the groups exhibited any toxic changes (Table 2). There were little significant changes in levels of serum GPT, GOT, or CRE in mice following each nSP70 treatment compared with the control group (Table 3). The toxicity test results were similar among nSP70, nSP70-N, and nSP70-C, which had different surface modifications. The same pattern was also observed between acetone/diethyl ether-pretreated and non-treated groups. The systemic toxicity examinations suggested that the application of each nSP70 induced no systemic side effects, although it was only conducted at one dosage concentration. Taken together, few adverse local and systemic effects were evident from histopathologic examination and blood tests, under the present experimental conditions.
| Intact skin | Acetone/diethyl ether-treated skin | |||||||
|---|---|---|---|---|---|---|---|---|
| nSP70 | nSP70-N | nSP70-C | — | nSP70 | nSP70-N | nSP70-C | — | |
| GPT (IU/L) | 17.8±5.5 | 18.5±5.0 | 9.5±1.8 | 11.5±2.4 | 28.6±6.6 | 19.5±7.8 | 33.2±6.9 | 18.2±6.3 |
| GOT (IU/L) | 56.1±6.1 | 106.1±53.0 | 48.2±11.5 | 62.6±25.3 | 69.6±16.9 | 69.0±18.0 | 68.2±23.9 | 61.4±7.3 |
| CRE (mg/dL) | 0.59±0.14 | 0.40±0.04 | 0.61±0.11 | 0.39±0.07 | 0.37±0.05 | 0.35±0.07 | 0.40±0.07 | 0.27±0.04 |
nSP70, nSP70-C, or nSP-70-N (each 125 µg/5 µL) was applied to skin with or without pretreatment of mixed acetone/diethyl ether solution for 28 d. Twenty-four hours after the last application, serum was collected to measure the GPT, GOT, and CRE concentrations. The data represent the mean±S.E. of five mice.
nSP70 showed in vitro cytotoxicity against the skin-derived keratinocyte cell line at the indicated concentration,9) so it could potentially cause tissue injury in vivo. In this experiment, it induced little local and systemic adverse reaction. However, it is important to understand the maximum dose resulting in toxicity, i.e., the severely toxic dose and the influence of particle sizes smaller than 70 nm. In addition, some cosmetics and sunscreens contain NMs, meaning the skin may be exposed to nSPs every day. Further study should focus on dosing at the severely toxic dose, whether nSP70 is actually toxic, and the highest nSP70 dosage not evidently resulting in lethality or life-threatening toxicities during extended application periods.
NMs are widely used in industry, and are being increasingly applied in pharmaceuticals and cosmetics. Careful dissection of NM-skin surface interactions will be important for assessing possible risks, and the effect of intended and unintended exposure on individuals with local or widespread skin barrier impairment. Examples include patients with atopic dermatitis and chronic eczema. A recent study suggested that exposure to TiO2 nanoparticles under skin barrier dysfunction/defects can exacerbate atopic dermatitis symptoms through T helper-2-biased immune responses.26) Further investigation to correlate penetration and toxicity with the type of skin barrier defect is required.
In conclusion, the present study demonstrated that decreasing particle size increased skin permeability, but with remarkably little skin irritation and systemic reactions. These findings will promote the manufacturing of products containing safe and useful NMs.
The authors declare no conflict of interest.