2016 Volume 39 Issue 8 Pages 1254-1262
2016 Volume 39 Issue 8 Pages 1254-1262
The effect of surface grafting with N-(carbonyl-methoxypolyethylene glycol-2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine (PEG2000-DSPE) onto three types of lipid nanocarriers, liposomes, niosomes and solid lipid nanoparticles (SLNs) on the skin penetration of sodium fluorescein (NaFI) was investigated. Confocal laser scanning microscopy (CLSM) was used to visualize the penetration pathways. Fourier transform infrared spectroscopy (FT-IR) was used to determine the skin hydration. The results showed that the physicochemical properties of each nanocarrier were modified after PEG grafting. In the skin penetration study, PEG grafting increased the flux of NaFI-loaded PEGylated liposomes and significantly decreased the flux of NaFI-loaded PEGylated niosomes and NaFI-loaded PEGylated SLNs. The skin deposition study and CLSM images showed that the intact liposome vesicles permeated into the skin. The niosomes and SLNs had little or no vesicles in the skin, suggesting that NaFI may have been released from these nanocarriers before permeation. Additionally, the fluorescent CLSM images of the SLNs showed that NaFI deposited along the length of hair follicles inside the skin, indicating that the skin penetration route may be through the transfollicular pathway. For the PEGylated nanocarriers, the PEGylated liposomes had higher fluorescence intensities than the non-PEGylated liposomes, indicating higher NaFI concentrations. The PEGylated niosomes and PEGylated SLNs had lower fluorescence intensities than those of the non-PEG modified niosomes and SLNs. For FT-IR results, PEGylated liposomes increased the skin hydration, while the grafting PEG onto niosomes and SLN surfaces decreased the skin hydration. This study showed that the surface grafting of PEG onto various nanocarriers affected the skin transport of NaFI.
Transdermal routes provide a controlled and non-invasive method of drug delivery. The outermost layer of skin, the stratum corneum, is a major protective barrier against the ingress of xenobiotics and controls the rate of water loss from the body. Therefore, drug transports via simple vehicles are unable to achieve therapeutic drug concentrations. Many strategies for enhancing skin penetration have been developed, such as chemical penetration, supersaturated systems, vesicles or physical mechanisms. A few examples of these strategies include the use of prodrugs, iontophoresis, electroporation, and ultrasound.1)
Lipid-based nanocarriers are the most sought after devices for topical and transdermal delivery applications. These nanocarriers include liposomes, niosomes, ethosomes, transferosomes, solid lipid nanoparticles (SLNs) and nanostructure lipid carriers (NLCs) have been extensively studied for the transdermal delivery of drugs. Liposomes have the potential to be drug-carrier systems for transdermal delivery. They contain amphipathic phospholipids arranged in one or more concentric bilayers enclosing an equal number of aqueous compartments. Hydrophilic agents can be entrapped within the inner aqueous sphere and lipophilic agents can intercalate in the lipid bilayers. Liposomes have been extensively studied for transdermal applications due to their similarity to biological membranes, ability to interact with similar lipids in skin, and decreased systemic absorption.2,3)
Niosomes, another lipid vesicle formulation, are similarly prepared and have the same functional and physicochemical properties as liposomes, such as the bilayer structure. However, niosomes differ from liposomes in their chemical structure. Niosomes are made of cholesterol and hydrated non-ionic surfactants and as such, have greater stability and lack many of the disadvantages associated with liposomes, i.e., high cost, low availability, and various purity issues commonly associated with phospholipids. Vesicular nanocarriers have been used as potential transdermal drug delivery systems due to their enhanced penetration capabilities, localized depots for sustained drug release, and rate-limiting membranes for the modulation of systemic absorption of drugs via the skin.4)
SLNs have been reported as alternatives to emulsions, liposomes, microparticles, and their polymeric counterparts for various application routes due to various advantages, such as the ability to incorporate lipophilic and hydrophilic drugs, improved physical stability, low costs relative to liposomes and easy scale-up and manufacturing potential.5,6) SLNs are colloidal nanocarriers system consisting of surfactant-coated, high melting point lipid nanoparticles (including high melting point glycerides or waxes).7) The small particle sizes of SLNs enable their close contact with the stratum corneum and thereby enhances the amount of encapsulated agent penetrating into the skin.8) Moreover, SLNs form occlusive films and have enhanced drug permeation.9)
Surface modifications of lipid nanoparticles using polyethylene glycol (PEG) derivatives have been reported to reduce the uptake of the nanoparticles by the reticuloendothelial system (RES). The behavior of PEGylated liposomes depends on the characteristics and properties of the specific PEG linked to the surface. The molecular mass of the polymer, as well as the graft density, determine the degree of surface coverage and the distance between graft sites. In most cases, longer-chain PEGs have produced the greatest improvements in blood residence time. Allen et al. reported that blood levels were higher for PEG liposomes with longer molecular weights (MW) of PEG (i.e., PEG2000 and PEG5000) than for liposomes containing PEG-lipids with a shorter chain of PEG (i.e., PEG750 and PEG120). The presence of PEG2000 doubled the amount of lipid remaining in the plasma compared to formulations containing PEG350 to PEG750.10) Moreover, N-(carbonyl-methoxypolyethylene glycol-2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine, sodium salt (PEG2000-DSPEs) are available as a commercial product and popularly used in PEGylation as a targeting carrier. PEG2000-DSPEs are amphiphilic molecules composed of hydrophobic phospholipids anchored in a bilayer of vesicles. The hydrophilic PEG chains extend out into aqueous media and serve as an outer shell.11) PEG-modified vesicles have been shown to have improved colloidal stability due to their altered physicochemical properties.12,13) However, very few studies have reported using PEGylated nanoparticles in transdermal drug delivery. Jain et al. reported that a topical application of PEGylated surfactant-containing liposomes increased the skin permeation of zidovudine by binding water molecules and increasing the hydration of the stratum corneum barrier.14) Knudsen et al. reported that calcipotriol-loaded liposomes with 1 mol% PEG significantly increased the accumulation of calcipotriol in skin and hair follicles when compared with other PEGylated liposome formulations.15)
The aim of this study was to investigate the effects of grafting PEG onto the surfaces of three different lipid nanocarriers on the skin transport of sodium fluorescein (NaFI). The surfaces of liposomes, niosomes, and SLNs were modified with PEG2000-DSPE. NaFI (C20H10Na2O5, MW 376 Da, log P=−1.52) was used as a model hydrophilic compound. Physicochemical characterizations were evaluated to compare the different lipid nanocarriers. In vitro skin penetration experiments and differential tape stripping techniques were performed to analyze the permeation of NaFI into skin. Confocal laser scanning microscopy (CLSM) was used to visualize the skin penetration pathway and Fourier transform infrared spectroscopy (FT-IR) was used to determine the skin hydration.
Egg phosphatidylcholine (PC) and Na-salt PEG2000-DSPE were purchased from Lipoid GmbH, Ludwigshafen, Germany. Cholesterol (Chol) was purchased from Carlo Erba Reagent, Ronado, Italy. Tween 20® was purchased from Ajax Finechem, Auckland, New Zealand. Tween 80® was purchased from the NOF Corporation (Osaka, Japan). Cetyl palmitate (CP) was purchased from SABO SpA (Levate, Italy). Sodium fluorescein (NaFI), d-limonene, and Span 20 were purchased from Sigma-Aldrich, MO, U.S.A. Lissamine™ rhodamine B 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine triethylammonium salt (Rh-PE) was purchased from Invitrogen, CA, U.S.A.
Nanocarrier FormulationsLiposomes PreparationThe liposome formulations contained controlled amounts of PC and Chol and various amounts of PEG2000-DSPE at molar ratios of 10 : 2 : 0, 10 : 2 : 0.6, 10 : 2 : 1.2, 10 : 2 : 3, and 10 : 2 : 6 mM as shown in Table 1. Liposomes were prepared using thin film hydration and sonication method. Briefly, a mixture of PC and Chol dissolved in chloroform–methanol (2 : 1, v/v) was added to a test tube, and nitrogen gas was used to evaporate the solvent. The lipid film was placed in a desiccator until completely dry (6 h). Then, NaFI dissolved in phosphate-buffered saline (PBS; pH 7.4) was added to the lipid film to produce hydrated liposome vesicles. This liposome dispersion was probe-sonicated in an ice-bath for 30 min to reduce the size of the liposomes. Excess lipids were separated from the vesicle formulation by centrifugation at 15000 rpm at 4°C for 15 min. The supernatant was collected for characterization.
| Formulations | PC | Cholesterol | Span 20 | CP | Tween 80 | PEG2000-DSPE | NaFI |
|---|---|---|---|---|---|---|---|
| CL | 0.773 | 0.077 | — | — | — | 0.000 | 0.2 |
| PL1 | 0.773 | 0.077 | — | — | — | 0.015 | 0.2 |
| PL2 | 0.773 | 0.077 | — | — | — | 0.030 | 0.2 |
| PL3 | 0.773 | 0.077 | — | — | — | 0.075 | 0.2 |
| PL4 | 0.773 | 0.077 | — | — | — | 0.150 | 0.2 |
| NI | — | 0.193 | 0.173 | — | — | 0.000 | 0.2 |
| NIP1 | — | 0.193 | 0.173 | — | — | 0.015 | 0.2 |
| NIP2 | — | 0.193 | 0.173 | — | — | 0.030 | 0.2 |
| NIP3 | — | 0.193 | 0.173 | — | — | 0.075 | 0.2 |
| NIP4 | — | 0.193 | 0.173 | — | — | 0.150 | 0.2 |
| SLN | 0.12 | — | — | 3 | 0.8 | 0.000 | 0.2 |
| SLNP1 | 0.12 | — | — | 3 | 0.8 | 0.015 | 0.2 |
| SLNP2 | 0.12 | — | — | 3 | 0.8 | 0.030 | 0.2 |
| SLNP3 | 0.12 | — | — | 3 | 0.8 | 0.075 | 0.2 |
| SLNP4 | 0.12 | — | — | 3 | 0.8 | 0.150 | 0.2 |
Abbreviations: PC, phosphatidylcholine; Chol, cholesterol; CP, cetyl palmitate; PEG2000-DSPE, N-(carbonyl-methoxypolyethylen glycol-2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine, sodium salt; NaFI, sodium fluorescein; CL, conventional liposomes; PL, PEGylated liposomes; NI, niosomes; NIP, PEGylated niosomes; SLN, solid lipid nanoparticles; SLNP, PEGylated solid lipid nanoparticles.
The niosome formulations contained controlled amounts of non-ionic surfactant (Span 20) and Chol and various amounts of PEG2000-DSPE at molar ratios of 5 : 5 : 0, 5 : 5 : 0.6, 5 : 5 : 1.2, 5 : 5 : 3, and 5 : 5 : 6 mM. The niosomes were prepared with the same method used for the liposomes. Briefly, a mixture of Span 20, Chol and various amount of PEG2000-DSPE dissolved in an ethanol–chloroform mixture (1 : 1, v/v) was placed in a desiccator, and the solvent was evaporated (6 h). Then, a NaFI solution was added to the lipid film to produce hydrated niosome vesicles. This dispersion was probe-sonicated in an ice-bath for 30 min to reduce the particle size. Excess lipids were separated from the vesicle formulation by centrifugation at 15000 rpm at 4°C for 15 min. The supernatant was collected for characterization.
SLNs PreparationThe SLN formulations were prepared using the de-novo emulsification method. The composition of the oil phase consisted of controlled amounts of cetyl palmitate and PC and various amounts of PEG2000-DSPE (0, 0.6, 1.2, 3, 6 mM). For the aqueous phase, NaFI and Tween 80® were dissolved in PBS. The oil and aqueous phases were heated at 65±5°C. Then, the aqueous phase was added to the oil phase under magnetic stirring at 14000 rpm for 5 min. The emulsions were probe-sonicated for 15 min to reduce the particle size and were then filtered through a 0.45-µm filter to remove any precipitates.
Characterization of Nanocarrier FormulationsParticle Size and Zeta Potential AnalysisEach formulation was diluted 1 : 10 with water and measured for size distribution and zeta potential, using a Dynamic Light Scattering (DLS) particle size analyzer (Zetasizer Nano-ZS, Malvern Instrument, Worcestershire, U.K.) with a 4 mW He–Ne laser at a scattering angle of 173°. All measurements were performed in triplicate.
Entrapment Efficiency (%EE) and Loading Efficiency (%LE)The nanocarrier dispersion (0.5 mL) was placed in an ultrafiltration tube with a molecular weight cutoff of 3000 Da (Microcon YM-3; Minipore, Billerica, MA, U.S.A.) and centrifuged at 4°C at 10000×g for 60 min. The filtrate was discarded, and 0.25 mL of PBS was added to the retentate before further centrifugation at 4°C at 10000×g for 40 min. The collected NaFI-loaded nanocarriers in the retentate were then disrupted with 0.2 mL of 0.1% weight per volume (w/v) Triton X-100 (for liposomes and niosomes) or isopropyl alcohol (for SLNs) and centrifuged at 4°C at 10000×g for 10 min. The NaFI contents of the supernatants were determined by fluorescence analysis.
The drug %EE and %LE were calculated with Eqs. 1 and 2:
 | (1) |
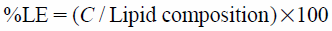 | (2) |
FT-IR spectrophotometer was used for characterized the stratum corneum at a molecular level. The stratum corneum layer was separated from the epidermis using trypsin digestion method. The whole skin (stratum corneum side facing upward) was placed on 0.5% w/v trypsin solution in water for 6 h at 37°C and then carefully removed stratum corneum sheet from viable epidermis. Stratum corneum sheets were rinsed with distilled water and dried in a desiccator till completely dried. Nanocarrier formulations were applied on stratum corneum sheets for 4 h at 32°C. The treated sheets were dried and investigated by FT-IR spectrophotometer (NICOLET4700; Thermo Electron Corporation, Madison, WI, U.S.A.) between 4000 and 1000 cm−1. All data were analyzed by version 8 of OMNIC software (Thermo Electron Corporation).
In Vitro Skin Penetration StudyAbdominal porcine skin was taken from intrapartum stillborn animals from a farm in Nakhon Pathom. Subcutaneous fat was carefully removed using medical scissors and surgical blades (skin thickness of 0.6–0.7 mm). Samples were frozen at −20°C until use and thawed at room temperature in PBS prior to experiments.
Franz diffusion cells were used to test the permeation of NaFI through porcine skin. The receptor compartment of each cell was filled with 6 mL of PBS at 32°C under 500 rpm stirring. Approximately 2 mL of NaFI-loaded nanocarrier formulations were applied to the skin in the donor compartment. Samples were taken at predetermined times (1, 2, 4, 6, 8, 24 h); 0.5 mL of the receiver medium was withdrawn for analysis using fluorescence detection, and the same volume of PBS was added to the receiver compartment to maintain a constant volume. Each sample was analyzed in triplicate.
The cumulative amount of nanocarriers permeating into the skin samples was plotted against time, and the steady-state flux was determined as the slope of the linear portion of the plot. The lag time was obtained by extrapolating the linear portion of the penetration profile to the abscissa. The skin permeation of model drug was analyzed using a mathematical model based on Fick’s law of diffusion (Eq. 3):
 | (3) |
The enhancement ratio (ER) was calculated as:
 | (4) |
After the in vitro permeation studies at 8 h, tape stripping was performed to collect the stratum corneum for each formulation. The skin was washed with PBS, blotted dry with soft tissue, and visible hair was removed with scissors. The adhesive tape (3 M, Hutchinson, MN, U.S.A.) was pressed onto the skin (1.96 cm2). Approximately 20 strips were applied, quickly removed and collected. After stripping, the skin was cut into small pieces. The collected stratum corneum and remaining skin (epidermis and dermis) were added to 20 mL of PBS, probe-sonicated for 20 min, and centrifuged for 10 min at 12000 rpm. The NaFI-containing stratum corneum, epidermis and dermis were analyzed using fluorescence detection. Each sample was analyzed in triplicate.
Fluorescence AnalysisSamples (100 µL) were pipetted into an opaque 96-well plate and analyzed using a fluorescence spectrophotometer (FusionTM Universal Microplate Analyzer, Packard Instrument Company, Inc., Downers Grove, IL, U.S.A.). The excitation wavelength was 485 nm, and the emission wavelength was 535 nm. Each sample was measured in triplicate.
CLSM StudyAfter running the in vitro skin penetration assays for 4 h using 0.1 mM rhodamine B-labeled liposomes, niosomes or SLNs, whole skins were sectioned using a cryostat (Leica 1850, Leica Instrument). Each skin sample was mounted on a metal sample holder with frozen section medium (Neg50, Microm International, Waldorf, Germany). The frozen skins were sectioned into 10-µm thick slices and mounted with mounting medium and covered with a cover slip on glass microscope slides. Confocal images were obtained using the ×10 objective lens of an inverted Zeiss LSM 510 META microscope (Carl Zeiss, Jena, Germany) with a He–Ne laser (543 nm excitation wavelength; 580 nm emission wavelength), an Ar laser (488 nm excitation wavelength; 514 nm emission wavelength) and a diode laser (358 nm excitation wavelength; 461 nm emission wavelength).
Statistical AnalysisAll measurements were collected in triplicate. Values are expressed as the mean±standard deviation (S.D.). Significance was analyzed using one-way ANOVA followed by Tukey’s post hoc test. The significance level was set at p<0.05.
The physicochemical characteristics of the different lipid nanocarrier formulations are presented in Table 2. The particle sizes of all formulations were between 70 and 228 nm with a narrow size distribution (polydispersity index; PDI <0.3). For the liposome formulations, the average particle sizes decreased in the order conventional liposomes (CL) >PEGylated liposome 1 (PL1)>PL3>PL4>PL2. The grafting of PEG onto the liposome surfaces significantly reduced the particle sizes of the liposomes; PEGylated PL2 showed the smallest particle size. The average particle sizes of niosomes decreased in the order niosome (NI)>PEGylated niosomes 1 (NIP1)> NIP2>NIP3>NIP4. The lipid components of the PEG2000-DSPE molecule intercalated into the liposome and niosome lipid bilayers, while the hydrophilic components, PEG2000, were outside the vesicles. With such an orientation, the PEG molecules strongly reduced attractive van der Waals forces and increased repulsive force (steric, electrostatic, and hydration).16) The zeta potentials of the liposome formulations were between −8.74 and −14.1 mV. Because the pH values of the liposome formulations (pH 7.4) was higher than the isoelectric point (pI) of PC (approximately 6 to 6.7) and because PC is a zwitterionic compound, the PC vesicles exhibited negative charges.17) The zeta potentials of the niosome formulations were between −17.93 to −35.2 mV. PEGylation significantly decreased the negative surface charge on the niosomes. Okore et al. reported that the surface negative charge of poly(methoxy-polyethyleneglycol cyanoacrylate-co-n-hexadecyl cyanoacrylate (PEG-PHDAC) niosomes dramatically decreased as the PEG chain length and concentration ratio increased. Furthermore, Okore et al. found that the magnitude of the surface charge was dependent on the acidic or basic strength of the surface groups and on the pH of the solution. For a solution of pH 7.4, a basic surface takes on a positive charge18); thus, a PEG-modified surface may reduce the negative surface charge of niosomes.
| Formulations | Particle size (nm) | PDI | Zeta potential (mV) | %EE | %LE |
|---|---|---|---|---|---|
| CL | 106.43±0.40 | 0.25±0.01 | −9.06±0.58 | 17.08±0.60 | 4.52±0.20 |
| PL1 | 71.69±0.60* | 0.24±0.01 | −9.73±0.30* | 27.81±0.09* | 6.90±0.02* |
| PL2 | 68.82±0.60* | 0.22±0.01 | −8.74±0.2 | 21.74±2.5* | 5.31±0.36 |
| PL3 | 71.35±0.30* | 0.29±0.01 | −14.10±0.4* | 26.14±0.14* | 6.51±0.03* |
| PL4 | 69.91±0.60* | 0.18±0.01 | −9.09±1.00 | 22.94±1.70* | 5.73±0.40 |
| NI | 228.07±0.60 | 0.20±0.01 | −35.20±1.30 | 40.87±0.50 | 22.34±0.30 |
| NIP1 | 163.43±1.70* | 0.28±0.01 | −24.23±0.60* | 28.97±7.60* | 15.84±4.20* |
| NIP2 | 129.43±0.30* | 0.24±0.01 | −21.23±0.90* | 31.76±4.47 | 17.36±2.40 |
| NIP3 | 125.13±0.10* | 0.26±0.01 | −17.93±0.60* | 28.64±3.50* | 15.66±1.90* |
| NIP4 | 105.16±5.50* | 0.27±0.01 | −19.30±0.60* | 33.16±2.30 | 15.66±1.90 |
| SLN | 142.17±1.80 | 0.15±0.01 | −25.23±1.00 | 45.11±2.10 | 2.80±0.10 |
| SLNP1 | 199.97±1.20* | 0.17±0.01 | −11.27±0.60* | 44.25±0.60 | 1.47±0.02* |
| SLNP2 | 197.33±1.90* | 0.17±0.01 | −11.87±0.90* | 51.49±0.29* | 1.72±0.01* |
| SLNP3 | 202.13±0.60* | 0.15±0.01 | −15.63±1.20* | 45.19±0.04 | 1.50±0.01* |
| SLNP4 | 197.03±1.50* | 0.18±0.01 | −16.47±0.40* | 56.47±0.95* | 1.88±0.03* |
Each value represents the mean±S.D. (n=3). * indicates significant difference between group (p<0.05). Abbreviations: CL, conventional liposomes; PL, PEGylated liposomes; NI, niosomes; NIP, PEGylated niosomes; SLN, solid lipid nanoparticles; SLNP, PEGylated solid lipid nanoparticles; PDI, polydispersity index; EE, entrapment efficiency; LE, loading efficiency.
For SLNs, the average particle sizes decreased in the order SLNP3>SLNP1>SLNP2>SLNP4>SLN. The presence of a PEG brush layer over the surface of nanoparticles significantly increased the nanoparticle size and decreased the zeta potential.13,19) However, there were no significant differences in the particle sizes and zeta potentials when different PEG concentrations were used.
%EE and %LEAs shown in Table 2, the %EE of the liposome formulations was 17 to 28%. The grafting of PEG onto the liposome surfaces exhibited a higher %EE than that for CL. The polarity of the bilayer vesicles in the presence of PEGylated-lipids was more than that of the non-PEG modified liposomes. Thus, PEGylation increased the incorporation efficiency of hydrophilic drugs.15,20)
For the niosome formulations, the %EE was 29 to 41%. The %EE of the niosomes was higher than that of the PEGylated niosomes (NIP1-4), suggesting that the PEG chain may make the niosome structure more rigid and decreased drug entrapment. The presence of cholesterol decreased the niosome bilayer fluidity but increased the drug loading efficiency. Additionally, the affinity of the drug for the niosome material, the thickness of the niosome bilayer, drug solubility in water and drug–drug interactions affected the drug loading efficiency.21)
SLN formulations showed the highest %EE, but lowest %LE. SLN formulations have higher lipid compositions than other formulations, therefore more NaFI can be entrapped. The %EE of SLNP4, the SLN with the highest PEG concentration, was significantly higher than non-PEG modified SLNs, suggesting that PEG molecules covalently attached to the nanoparticle surface and provided a hydrophilic steric barrier around the SLNs.22) This barrier likely helped to retain the hydrophilic drug within the SLNs more than the other formulations.
FT-IR StudyFT-IR spectroscopy was used to determine changes in hydration of the stratum corneum of the skin. The intensity ratio of the amide I/amide II band in FT-IR is called the moisture factor, and hydration increases the ratio between amide-I and amide-II peaks in FT-IR.23,24) Figure 1 shows the intensity ratio of the amide I (ca. 1652.3 cm−1) /amide II (ca. 1541.8 cm−1) band in FT-IR of different lipid nanocarriers. PL2 exhibited a higher intensity ratio than CL, indicating that the hydrophilic PEG molecule might have bound the water molecules and penetrated the skin leading to increase hydration of the stratum corneum. The intensity ratios of the amide I/amide II of the stratum corneum treated with PEG niosomes and PEG-SLN were lower than PL. Cholesterol in niosomes can act as emollients to fill in spaces between corneocytes on the skin surface and increase skin hydration.25) However, PEGylated niosomes decreased skin hydration, suggesting that the steric barrier of PEG molecules around the niosome surfaces might stabilize the niosome vesicles and reduce the cholesterol released into the skin. SLNs can increase skin hydration due to the occlusive factor by film formation on the skin surface. The grafting of PEG molecules onto SLN surfaces could prevent particle agglomeration,6) leading to reduce film formation on the skin and decreased skin hydration, and thereby decrease the penetration of entrapped drug through the skin.

The amount of NaFI delivered from different lipid nanocarrier formulations into the skin plotted against time is shown in Fig. 2. The penetration parameters of the different NaFI-loaded lipid nanocarriers are shown in Table 3. The rate of absorption, or flux (J), of any substance across a barrier is proportional to its concentration difference across that barrier; the proportionality constant relating the flux to the concentration is the permeability coefficient (Kp).26) In this study, the lag times of all formulations were not significantly different. The NaFI-loaded lipid nanocarriers showed higher NaFI permeation through skin than NaFI solutions. The SLNs showed the highest NaFI permeation through the skin, suggesting that the SLNs can entrap NaFI more than the other formulations. The solid structure of lipid particles were more lipophilic and could closely contact the skin. Additionally, the lipid nanoparticles enabled the formation of a thin film on the skin, providing an occlusive effect, resulting in increased skin hydration, and consequently, the enhanced penetration of the drug into the skin.27) Therefore, it was anticipated that the formulation containing a greater amount of solid lipids would lead to greater skin penetration.

Each value represents the mean±S.D. (n=3).
| Formulations | Flux (μg/cm2/h) | ER | Lag time (h) | Kp (cm/h) (×10−6) |
|---|---|---|---|---|
| NaFI sol | 0.0058±0.00 | — | 0.54±0.19 | 2.90±0.95 |
| CL | 0.0078±0.00 | 1.34 | 1.00±0.35 | 3.90±2.91 |
| PL1 | 0.0258+0.00 | 4.45 | 0.50±0.10 | 12.89±1.46 |
| PL2 | 0.0639±0.05 | 11.02 | 0.86±0.00 | 32.00±25.53 |
| PL3 | 0.0620±0.03 | 10.69 | 0.70±0.26 | 31.00±13.50 |
| PL4 | 0.0244±0.00 | 4.21 | 0.40±0.20 | 12.22±1.33 |
| NI | 0.1035±0.08 | 17.84 | 0.58±0.33 | 51.77±41.06 |
| NIP1 | 0.0111±0.01 | 1.91 | 0.30±0.30 | 5.58±4.71 |
| NIP2 | 0.0433±0.01 | 7.47 | 0.85±0.30 | 20.00±5.12 |
| NIP3 | 0.0190±0.01 | 3.28 | 0.38±0.33 | 9.49±6.50 |
| NIP4 | 0.0314±0.01 | 5.41 | 0.35±0.35 | 15.72±3.81 |
| SLN | 0.1920±0.06 | 33.10 | 0.43±0.23 | 95.98±30.70 |
| SLNP1 | 0.0461±0.04* | 7.95 | 0.73±0.31 | 23.07±20.34* |
| SLNP2 | 0.0577±0.04* | 9.95 | 0.57±0.23 | 28.87±20.93* |
| SLNP3 | 0.0184±0.02* | 3.17 | 0.73±0.31 | 9.22±11.47* |
| SLNP4 | 0.0041±0.00* | 0.71 | 0.23±0.21 | 2.05±0.74* |
Each value represents the mean±S.D. (n=3.). * indicates significant difference between group (p<0.05). Abbreviations: CL, conventional liposomes; PL, PEGylated liposomes; NI, niosomes; NIP, PEGylated niosomes; SLN, solid lipid nanoparticles; SLNP, PEGylated solid lipid nanoparticles; ER, enhancement ratio; Kp, permeability coefficient.
For niosomes, a higher NaFI flux penetrate through the skin than the liposomes, suggesting that niosomes improved horny layer properties by reducing transepidermal water loss and increasing smoothness via the replenishment of lost skin lipids.28) Therefore, niosomes containing nonionic surfactants and cholesterol have been used as drug delivery systems as alternatives to conventional liposome-based delivery methods. Niosomes offer higher chemical and physical stabilities,29) a greater availability of surfactant classes,30) and improved penetrations of entrapped substances across the skin.31) Liposomes are of little or no value as carriers for transdermal drug delivery due to their inability to deeply penetrate skin. Due to the alteration to the stratum corneum lipid structure, vesicles remain confined to the stratum corneum layer.32)
The PEG-grafted liposome vesicles provided a higher transdermal flux than CL. The particle size of PL1–PL4 was smaller and the %EE of PL1–PL4 was higher than CL. Verma et al. reported that a hydrophilic fluorescent compound (carboxyfluorescein (CF)) loaded into the small liposome vesicles (120 nm) showed statistically enhanced penetration of CF into the skin as compared to larger ones (191, 377, 810 nm).33) Moreover, CF incorporated into the liposomes with higher %EE exhibits a higher penetration than those with lower %EE.34) Therefore, the particle size and %EE substantially affect the transdermal drug absorption of liposome vesicles. Skin hydration of PL2 formulation was higher than CL (Fig. 1), therefore skin hydration may also be a factor affecting the differences in skin penetration of NaFI in PEGylated liposomes and CL. The PEG-DSPE molecule may have increased skin penetration by binding to water molecules, increasing the hydration of the stratum corneum and enhancing the permeation across this barrier.15)
For the niosomes, the NaFI flux of the PEGylated niosomes was lower than that of non-PEGylated niosomes. However, the size and %EE of PEGylated niosomes was smaller than non-PEG niosomes. Junyaprasert et al. explained the effect of vesicle size and %EE of different niosome formulation entrapped ellagic acid (EA). They reported that the different penetration enhancement properties of different niosome formulations were caused by the particle size, %EE and interaction of the solubilizer in the niosomes with the skin.35) In this study, the drug released from vesicles before skin permeation, therefore skin hydration by niosomes might play an important factor affecting skin permeation. High skin hydration of both PEGylated and non-PEGylated niosomes was observed (Fig. 1). However, non-PEGylated niosomes provided higher skin hydration than PEGylated niosomes. This might be caused by the fact that the steric barrier of PEG molecules around the niosome surfaces stabilized the niosome vesicles, thus reducing the cholesterol released into the skin, thus reducing the skin hydration and leading to a decrease in skin permeation.15)
In the case of the SLNs, the particle size and the %EE of PEGylated SLNs (SLNP 2–4) were significantly higher than non-PEGylated SLNs, however the NaFI flux for the PEGylated SLNs significantly decreased when compared with that of non-PEGylated SLNs. The skin hydration of SLNP2 was significantly lower than SLN (Fig. 1). These results suggested that the increased skin hydration showed more effect on the enhanced skin permeation of NaFI in SLN formulation than the particle size and %EE. The PEG molecules changed the surface properties of the SLNs by altering the surface hydrophobicity of the particles and inducing a hydrophilic steric barrier around the SLNs.22) Therefore, the PEGylated SLNs likely reduced film formation on the skin and thereby decreased the penetration of entrapped drug through the skin.
In Vitro Tape StrippingTape stripping is commonly used to quantify drug penetration through skin and to examine the distribution of substances within the stratum corneum.36) As shown in Fig. 3, the amount of NaFI penetrating into the stratum corneum decreased in the following order: NI (3.68±0.18 µg)>NIP2 (3.25±0.68 µg)>CL (3.24±0.45 µg)>PL2 (3.22±0.39 µg)>SLNP2 (2.86±0.62 µg)>SLN (2.22±0.43 µg)>NaFI solution (1.89±0.52 µg). The NaFI penetration of the stratum corneum from lipid nanocarriers was significantly higher than from NaFI solution (p<0.05), but not significantly different between the PEG and the non-PEG modified nanoparticles. The amounts of NaFI in the stratum corneum from the niosomes and the liposomes were not significantly different. The SLNs showed lower NaFI deposition in the stratum corneum than the niosomes and liposomes. The amount of NaFI in the epidermis and dermis (ED) was in the order: PL2 (1.90±0.60 µg)>CL (1.46±0.16 µg)>NaFI solution (1.19±0.42 µg)>NI (1.11±0.09 µg)>NIP2 (0.79±0.02 µg)>SLN (0.79±0.25 µg)>SLNP2 (0.34±0.12 µg). NaFI deposits observed in the epidermis and dermis for all formulations were lower than the NaFI deposits in the stratum corneum.

Each value represents the mean±S.D. (n=3). * indicates significant difference between group (p<0.05).
As shown in Fig. 3(A), PL2 showed higher NaFI depositions in the epidermis and dermis than CL and the other formulations. Traditional liposomes containing phospholipids and cholesterol did not penetrate skin deeply. Instead, these liposomes remained confined in the stratum corneum.32) The PEG-modified liposome surface enhanced NaFI delivery into the deeper skin layers. PEG, a hydrophilic polymer conjugated with phospholipids, better hydrates the stratum corneum than phospholipids without PEG.15) For niosomes, the effects of PEG and non-PEG on the skin surface were not significantly different (Fig. 3(B)).
For the SLNs, Fig. 3(C) shows that the amount of NaFI deposited in the stratum corneum was higher than those in the epidermis and dermis layers. Although SLNs are known to have beneficial properties for topical drug therapy, including biocompatible ingredients, drug release modifications, skin adhesion, and film formation with the subsequent hydration of superficial skin layers, their penetration and permeation into and across deeper skin layers are restricted due to the barrier function of the stratum corneum.37) A few intact SLNs form an occlusive film on the top layer of the skin surface; these SLNs were observed in the first tape-stripped stratum corneum layer. Therefore, the observed enhanced drug permeation was likely due to particle occlusion properties.9) The PEG-grafted SLNs surface showed lower amounts of NaFI deposits in the epidermis and dermis than the non-PEG modified SLNs, suggesting that a hydrophilic steric barrier around the SLN surfaces may have reduced the ability for the entrapped NaFI to deeply penetrate into the skin layer.
CLSM StudyThe confocal images of sectioned skin samples obtained 4 h after the deposition of NaFI solution and NaFI-loaded-Rh-PE-labeled lipid nanocarriers are shown in Fig. 4. For the NaFI solution, NaFI only penetrated the top of stratum corneum layer. For the liposomes CL and PL, the red fluorescent-labeled membrane vesicles and green fluorescent NaFI penetrated the skin. While the skin treated with niosomes and SLNs showed the green fluorescence of NaFI but no value or a little red fluorescence of Rh-PE probed lipid nanocarriers, indicating that the niosomes and SLNs were unable to form vesicles within the skin. In these results, the phospholipid-based liposomes have the same properties as those of stratum corneum lipids, as their adhesion onto the skin surface and fusion or mixing with the lipid matrix of the stratum corneum has suggested.38) Moreover, the PEGylated liposomes showed higher fluorescent intensities for NaFI and vesicle probes than those of CL, suggesting that intact PL vesicles can act as NaFI vehicles for delivery to deep skin layers.

The image is divided in three parts, with (1) light gray fluorescence of NaFl, (2) gray fluorescence of Rh-PE and (3) overlay of (1) and (2). The scale bar represents 100 µm. All confocal images were obtained at a magnification of ×10. Symbols: (⇨, hair follicles) and (- - -, between epidermis and dermis layers).
The skin treated with niosomes and SLNs showed the green fluorescence of NaFI but no value or little red fluorescence of Rh-PE probed lipid nanocarriers, indicating that the niosomes and SLNs did not permeate the skin; only drugs permeated the skin surface. These results suggested that NaFI molecules were released from the nanocarriers before permeation through the skin. Additionally, a CLSM image of SLNs (Fig. 4(F)) shows that NaFI deposited along the length of hair follicles inside the skin more than the other formulations, indicating that the transfollicular pathway may be a penetration route for NaFI from SLNs into skin. In previous studies, particulate drug carriers, e.g., lipid nanoparticles, have been shown to penetrate and preferentially accumulate in hair follicles. This results in high local concentrations of a drug due to the lipophilic properties of the carriers leading to improved drug uptake via hair follicles.37,39) Therefore, the surface modification of SLNs using hydrophilic polymers may reduce NaFI penetration through hair follicles.
The flux of NaFI in nanocarries can be ranged from SLNs>niosomes>liposomes. These might be caused by differences in skin hydration. PEG grafting increased the flux of NaFI-loaded PEGylated liposomes, but significantly decreased the flux of NaFI-loaded PEGylated niosomes and NaFI-loaded PEGylated SLNs. In PEGylated liposomes, intact liposome vesicles permeated the skin. Not only do physicochemical properties (size and %EE), but also skin hydration play a major role in skin permeation. However, in PEGylatd niosomes and PEGylated SLNs, NaFI has been released from these nanocarriers before skin permeation. Therefore, skin hydration plays a more important role for skin permeation than the physicochemical properties (size and %EE) of niosomes and SLNs. The results indicated the significance of nanocarriers and the surface grafting onto nanocarriers on the skin permeation of the hydrophilic compound.
We gratefully acknowledge the financial support provided by the Thailand Research Funds through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0091/2554) and through the Basic Research Grant (Grant No. 5680016). We wish to thank the Division of Medical Molecular Biology (Department of Research and Development, Faculty of Medicine, Siriraj Hospital, Mahidol University) for providing access to their confocal laser scanning microscope (LSM 510 Meta, Zeiss, Jena, Germany).
The authors declare no conflict of Interest.