2016 Volume 39 Issue 9 Pages 1488-1495
2016 Volume 39 Issue 9 Pages 1488-1495
The effective cure for oral squamous cell carcinoma (OSCC) patients is challenging due late diagnosis and fatal metastasis. The standard diagnosis for OSCC often depends on the subjective interpretation of conventional histopathology. Additionally, there is no standard way for OSCC prognosis. Over the past decade, nano-mechanical stiffness has been considered as a quantitative measure for cancer diagnosis. Nevertheless, its application to OSCC diagnosis and prognosis is still in a primitive stage. In this study, we investigated whether the OSCC progression can be predicted by nano-mechanical properties in combination with biochemical properties, especially the epithelial–mesenchymal transition (EMT). Atomic force microscopy-based nano-mechanical measurements of three different OSCC cell lines—SCC-4, SCC-9, and SCC-15—were conducted together with biochemical analyses. The gradual upregulation of Snail2, N-cadherin, and vimentin and the simultaneous downregulation of E-cadherin were observed, and the degree of upregulation and downregulation was stronger in the order of the cell lines mentioned above. The strength of enhancement in migration was in the same order as well. Consistently, nano-mechanical stiffness was gradually decreased as the EMT progresses. These results suggest that the nano-mechanical assay could serve as a quantitative tool to predict the OSCC progression in the context of the EMT. Furthermore, we found that the upregulated vimentin, a major filamentous component of the cytoskeleton, may contribute to mechanical softening, which can be discerned from the role of actin filaments in mechanical stiffness. In conclusion, our combinational study proposes a novel way to elucidate the mechanism of OSCC progression and its therapeutic targets.
Oral squamous cell carcinoma (OSCC) is the most common neoplasm of the head and neck squamous cell carcinoma (HNSCC), which represents approximately 6% of all types of cancer. Also about half a million new cases are diagnosed every year.1) It is a highly invasive malignant tumor characterized by a high rate of metastasis to the cervical lymph nodes.2) Diagnosis and management of OSCC have improved by combining therapies such as radical surgery, radiotherapy and neo-adjuvant chemotherapy, but the overall 5-year survival rate is still below 50% because of late diagnosis and high rate of recurrence.3,4) Thus, it is highly desirable to understand the underlying mechanism governing metastatic dissemination of OSCC for the development of anticancer therapeutics.
The progression of OSCC is largely dependent on the epithelial–mesenchymal transition (EMT).5,6) Recent studies have reported that the EMT has been strongly associated with cancer cell stemness7,8) and chemoresistance to anti-cancer drugs,9–12) indicating that targeting EMT could be a good strategy to overcome cancer metastasis. Notably, transforming growth factor-β1 (TGF-β1) has been known to be a primary inducer of EMT both during developmental process and cancer progression.13) In TGF-β1-induced EMT signaling pathway, the universal features including the downregulation of E-cadherin and vimentin and N-cadherin are regarded as molecular hallmarks of EMT.14) Although extensive research has been focused on signaling networks responsible for the EMT, much remains to be understood regarding this complex and dynamic cellular process.
During the EMT, cancer cells undergo morphological changes from cuboidal epithelial cells to spindle-like mesenchymal cell phenotype. Such phenotypic features might be tightly regulated by the cytoskeletal rearrangement and contribute for the enhanced invasion and the acquisition of anti-cancer drug resistance, which were frequently noticed during the EMT.15–17) Over the past decade, nano-mechanical assays revealed that cancer cells experiences an extensive level of cytoskeletal reorganization that causes subsequent changes in mechanical properties such as motility, adhesion, and mechanical stiffness during the metastatic journey.18) In particular, cancer cells might adjust their mechanical properties as well as biochemical signals for the successful detachment of carcinoma cells from the epithelium and the subsequent invasion of the underlying stroma during the EMT.19) Nevertheless, little is known about the interplay between the adjustment of mechanical properties and the biochemical signaling pathway of the EMT.
Several tools such as micropipette aspiration,20) optical stretchers,21) and magnetic twisting cytometry22) were used in order to investigate mechanical properties of biological cells. However, advantages such as the adaptability to physiological environments and the ability to measure forces with pico-newton sensitivity from precisely controlled local domains make atomic force microscopy (AFM) one of the most widely used tools to characterize mechanical properties of cells.23) The decrease in the mechanical stiffness revealed by AFM-based biomechanics was suggested to be a promising marker for cancer diagnosis.18,24,25) However, the relationship between the mechanical stiffness and the metastatic potential of cancer cells has not been unequivocally established. Some studies reported the decrease in the mechanical stiffness as a mechano-phenotypic feature of metastatic cancer cells.26–29) On the contrary, the opposite correlation has been reported specially for prostate cancer cells.30–32) Since the mechanical stiffness was suggested as a diagnostic marker for oral cancer cells,33) several studies examined its possible use for prognosis.34,35) A recent study using AFM reported the reduction of mechanical stiffness in metastatic tongue squamous cells due to changes in actin filaments and microtubules.35)
There are many filamentous components such as microtubules, vimentins, and keratins in the cytoskeleton.18) Until recently, a substantial level reorganization of actin filaments was noticed as a major cause responsible for mechanical changes in cancer cells.25,36,37) However, only a few studies investigated the possible significance of other components such as keratins in cancer progression.38,39) Vimentin, one of the intermediate filaments, is known to be overexpressed in various epithelial cancers. It is a canonical marker of the EMT. There has been a lack of studies revealing the role of vimentin in changing the mechanical stiffness of cells. Recently, the interruption of vimentin was reported to cause mechanical alterations of cells.40) Nevertheless, detailed correlations among the EMT, the vimentin expression, and the mechanical stability during cancer progression have yet to be rectified.
In this study, we investigate the correlation between mechanical properties and metastatic progression of oral squamous cancer cell lines by focusing on the progression of the EMT. The TGF-β1 known as an EMT inducer was used in order to confirm this correlation. Our novel results obtained by the nano-indentation assay using AFM were presented together with the systematic biochemical assays about the EMT.
Antibodies for E-cadherin (#3195), N-cadherin (#4061), vimentin (#3932), Snail2 (#9585) and β-actin (#4970) were purchased from Cell Signaling Technology (Beverly, MA, U.S.A.). TGF-β1 was obtained from R&D Systems (Minneapolis, MN, U.S.A.).
Cell CultureThe human oral squamous cell carcinoma (OSCC) cell lines SCC-4, SCC-9, and SCC-15 were obtained from American Type Culture Collection (ATC C, Manassas, VA, U.S.A.). Cells were maintained in DMEM/F12 (HyClone, South Logan, UT, U.S.A.) supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin and cultured as monolayer at 37°C with 5% CO2 in a humidifier incubator.
Western Blot AnalysisTotal cell lysates were isolated from harvested cells, and the amount of protein was determined using BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, U.S.A.). Cells were harvested in the ice-cold lysis buffer (50 mM Tris–HCl pH 8.0, 150 mM NaCl, 1% NP40), and 30 µg of lysate per lane was resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. Following a transfer onto the polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, NJ, U.S.A.), the membrane was blocked with 5% skim milk in Tris-buffered saline with Tween 20 (TBS-T) and then proved with primary antibodies overnight at 4°C. After the binding of a species-specific secondary antibody, protein bands were visualized by adding SuperSignal® West Dura Extended Duration Substrate (Thermo Scientific, Waltham, MA, U.S.A.) and developed with LAS-3000 (FUJI, Japan) according to the instructions of the manufacturer.
Reverse Transcription (RT)-PCRTotal RNA was isolated from the cultured cells using TRIzol reagent (Invitrogen, Carlsbad, CA, U.S.A.) according to the manufacturer’s instructions. After quantifying with NanoDrop (Nyxor Biotech, France), 2 µg of DNase-treated RNA was used for cDNA synthesis using M-MLV Reverse transcriptase (Promega, Madison, WI, U.S.A.). The PCR reaction was performed using ExTaq polymerase (TaKaRa, Japan). The sequence for PCR primers is listed in Table 1.
| Gene | Sequences | |
|---|---|---|
| Snail1 | Forward | 5′-GAG GCG GTG GCA GAC TAG-3′ |
| Reverse | 5′-GAC ACA TCG GTC AGA CCA G-3′ | |
| Snail2 | Forward | 5′-CAT GCC TGT CAT ACC ACA AC-3′ |
| Reverse | 5′-GGT GTC AGA TGG AGG AGG G-3′ | |
| ZEB1 | Forward | 5′-TGC ACT GAG TGT GGA AAA GC-3′ |
| Reverse | 5′-TGG TGA TGC TGA AAG AGA CG-3′ | |
| Twist | Forward | 5′-CTC GGA CAA GCT GAG CAA GAT TCA GA-3′ |
| Reverse | 5′-CGT GAG CCA CAT AGC TGC AGC-3′ | |
| E-Cadherin | Forward | 5′-TGC CCA GAA AAT GAA AAA GG-3′ |
| Reverse | 5′-GGA TGA CAC AGC GTG AGA GA-3′ | |
| N-Cadherin | Forward | 5′-GAC AAT GCC CCT CAA GTG TT-3′ |
| Reverse | 5′-CCA TTA AGC CGA GTG ATG GT-3′ | |
| Vimentin | Forward | 5′-GAG AAC TTT GCC GTT GAA GC-3′ |
| Reverse | 5′-TCC AGC AGC TTC CTG TAG GT-3′ | |
| GAPDH | Forward | 5′-TCG ACA GTC AGC CGC ATC T-3′ |
| Reverse | 5′-CCG TTG ACT CCG ACC TTC A-3′ |
After fixation and permeabilization, three OSCC cell lines—SCC-4, SCC-9, and SCC-15 cells—were incubated with the primary antibody specific for vimentin and then incubated with the fluorescein isothiocyanate (FITC)-conjugated secondary antibody. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and imaged using a fluorescence microscope.
Migration AssayMigratory capacities of the cells were tested using a cell culture insert for 24 well plates with 8.0 µm pore (Corning, NY, U.S.A.). The cells were suspended in the culture media (1×105 cells/mL), and then 0.5 mL cell suspension (5×104 cells/mL) was added to the upper chambers. After incubating for 24 h, the culture media in the inserts were carefully removed, and the membrane containing the cells on the lower surface of inserts was fixed using methanol and stained with hematoxyline–eosin (H&E). The upper surface of the membrane was gently scrubbed with a cotton swab to remove cells. The stained cells were quantified from 5 randomly selected areas under a light microscope. Three independent experiments were performed, and the data are presented as the average number of migrated cells (% of SCC4).
AFM MeasurementsAll AFM measurements were taken with a MFP 3D® (Asylum Research, Santa Barbara, CA, U.S.A.) atomic force microscope equipped with a fluid cell and Bioheater™ operating at 37°C. Data collection was carried out only for the first three hours after the initial measurement to ensure the healthiness of the cells. V-Shaped silicon nitride cantilevers modified with polystyrene beads were used in order to obtain a well-defined contact area and to reduce the stress from otherwise sharp AFM tips. Cantilever spring constants (typically 0.01 N/m) were calibrated by the thermal noise fluctuations method. The radius of the modified probe tip (1.5–4 µm) was accurately determined from AFM topographic images prior to the experiments. In order to obtain the elastic moduli, nano-indentation experiments were performed by acquiring force–distance (f–d) curves with a one-second time interval, i.e., 1 Hz, with trigger forces ranging from 500 pN to 1000 pN. Measurement points were selected in a region over the center of a single cell identified by real-time images obtained with an inverted optical microscope (IX-81®, Olympus microscope, Tokyo, Japan) on which the atomic force microscope was installed. As a control experiment, one f–d curve was obtained from a hard glass slide before taking measurements from the cells in order to verify the linear increase in force (f) as a function of the z-scanner displacement (d).
Calculation of Elastic ConstantsIn order to determine the elastic moduli, obtained f–d curves were converted to the corresponding f–δ curves. Force f applied to the cell was calculated by multiplying the spring constant k with the cantilever deflection. The Hertz model was used to determine the elastic moduli from f–δ curves. According to the Hertz model (Eq. 1), a f–δ curve can be converted to a curve of elastic constant K=E/(1−ν2) versus the dimensionless quantity δ/R, where the R is the radius of the spherical tip and ν is the Poisson ratio. The elastic constant K should remain nearly constant as δ/R varied for a linear, homogenous sample.
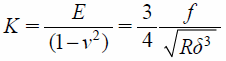 | (1) |
Data were expressed as the mean±standard error of the mean (S.E.M.). Statistical significance was identified by Student’s t-test or one-way ANOVA for the difference among observed pairs.
The morphological difference of oral squamous cell carcinoma cell lines—SCC-4, SCC-9, and SCC-15—was first examined. Our data revealed that SCC-9 and SCC-15 cells showed spindle-shaped mesenchymal morphology whereas SCC-4 cells exhibited cuboidal epithelial morphology (Fig. 1A). Moreover, the immunofluorescence data showed a more enhanced expression of vimentin, a marker of mesenchymal phenotype, in SCC-9 and SCC-15 cells compared with SCC-4 cells (Fig. 1B). This phenotype was further confirmed by RT-PCR and Western blot analysis of the EMT markers. We observed the upregulation of Snail2, ZEB2, N-cadherin, and vimentin and the simultaneous downregulation of E-cadherin (Figs. 1C, D). Based on these findings, we concluded that three OSCC cells have distinct characteristics with different EMT phenotypes.

(A) Morphological differences among SCC-4, SCC-9, and SCC-15 cells were evaluated using a light microscope. (B) Immunofluorescence images showing the expression of an EMT marker, vimentin in three OSCC cells. Images show representative portions of cells for vimentin (green) and DAPI (blue) for nuclear counterstaining. (C) The expression of EMT markers was analyzed by RT-PCR. GAPDH was used as a loading control. (D) The protein expression of EMT markers was analyzed by Western blot analysis using indicated antibodies. β-Actin was used as a loading control.
Cells undergoing the EMT acquire a mesenchymal phenotype and increased motility which further contribute to metastatic progression. Vimentin expression, which could accompany a cytoskeletal reorganization, has been known to promote tumor cell migrations and invasions. Thus, we checked migratory properties of three OSCC cell lines. Our data revealed that the ability to migrate was significantly enhanced in the order of SCC-4, SCC-9, and SCC-15 (Figs. 2A, B). These findings are consistent with the above mentioned morphological and molecular results showing the different EMT phenotypes for observed OSCC cell lines.

(A) The representative photographs for migration of SCC-4, SCC-9, and SCC-15 cells. (B) The percentage of SCC-4 and SCC-15 cells that migrated compared with SCC-4 cells. Live cells that migrated to the lower surface were fixed, stained, and counted using a light microscope. Five random areas were scanned on the lower surface of the membrane in the Boyden chamber. Error bars represent the mean±S.D. (**p<0.01, ***p<0.001).
The mechanical compliance of oral squamous cell carcinoma cell lines, SCC-4, SCC-9, and SCC-15 was determined from the AFM indentation experiments. Representative force–indentation (f–δ) curves obtained at cell centers are shown in Figs. 3A to C. For the same trigger force of 1.0 nN, the obtained maximum indentation significantly increased in the order of SCC-4, SCC-9, and SCC-15, indicating gradual softening of the mechanical stiffness. The quantitative determination of the mechanical stiffness, i.e., elastic constants was performed using the Hertz model. The experimental data (dots) agreed well with the Hertz model (solid lines) so that the elastic constants K could be successfully determined. All observed cells displayed no significant variation in the elastic constants within the observed trigger force, except for the elastic constants measured from SCC-4 cells with 500 pN of the trigger force (Fig. 4A). This result validated that the elastic constants were measured in shallow indentation ranges thereby satisfying the assumptions of the Hertz model. The average elastic constants (mean±S.E.) were 1122±148, 553±59, and 208±29 Pa for SCC-4 (n=18), SCC-9 (n=19), and SCC-15 (n=23) cells, respectively (p<0.001) (Fig. 4B). n Represents the number of cells investigated for each cell line for the statistical analysis. As expected from f–δ curves, a significant decrease in elastic constants was confirmed in SCC-9 and SCC-15 cells compared with SCC-4 cells.

(A–C) Typical f–d curves obtained at the centers of SCC-4 (A), SCC-9 (B), and SCC-15 (C) cells with the trigger force of 1 nN. The dark solid lines represent fits to the Hertz model. The gradual increase in indentations were observed from SCC-4 to SCC-15 cells for the observed trigger forces.

Over the range of applied trigger forces except 500 pN for the SCC4 cells, the oral cancer cell lines display the homogeneous nature of mechanical compliances, validating the applicability of the Hertz model to determine the elastic constants. A clear shift to the higher elastic constants was observed in SCC-4 and SCC-9 cells compared with SCC-15 cells. (B) The average elastic constants determined from the cell centers of SCC-4, SCC-9, and SCC-15 cells. Gradual increases in elastic constants were observed from SCC-15 to SCC-4 cells (p<0.001). There is no statistical difference in the trigger forces applied to observe cell lines.
The histogram in Figs. 5A to C showed the distribution of elastic constants from all observed cell lines. Interestingly, the width in the distribution of elastic constants measured for each cell line gradually decreased in the order of SCC-4, SCC-9, and SCC-15 cells. The elastic constants of SCC-4 cells showed in the widest range of up to 4.5 kPa (Fig. 5A) while there was no occurrence of Young’s moduli greater than 2 kPa for SCC-15 cells (Fig. 5C). The mechanical heterogeneity represented by the distribution of elastic constants is an intrinsic characteristic of each cell line.
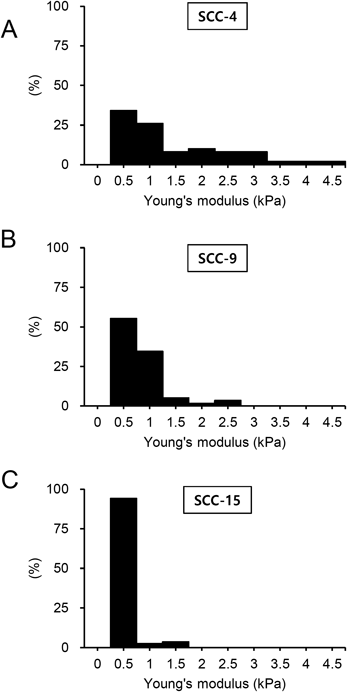
The distribution range of the elastic constants increased in the order of SCC-4, SCC-9, and SCC-15 cell lines. The elastic constants of SCC-4 cells show the most significant shift toward greater values compared with SCC-9 and SCC-15 cells.
In order to verify the correlation between mechanical compliance and EMT process, we performed the experiments using TGF-β1 known as a primary EMT inducer. We investigated whether the treatment with TGF-β1 in SCC-4 cells could accompany the significant decrease in elastic constants as well as the EMT signatures found in protein expressions. When treated with 2.5–5 ng/mL TGF-β1 for 24 h, SCC-4 cells showed the loss of epithelial marker, E-cadherin and the gain of mesenchymal markers, Snail2, N-cadherin, and vimentin (Fig. 6A). We also found from the AFM measurements that the treatment of TGF-β1 (5 ng/mL) for 24 h induced the loss of incidences showing the Young’s moduli greater than 2 kPa and thus the narrower distribution of Young’s moduli (Fig. 6B). Consequently, the average Young’s moduli of TGF-β1-treated cells were significantly lower than those of non-treated cells (Fig. 6C). Combined together, these results imply that the enhanced mechanical compliance is highly correlated with the EMT signatures observed from protein markers in OSCCs.

Serum-starved SCC-4 cells for 2 h were treated with 2.5–5 ng/mL TGF-β1 for 24 h. (A) The protein expression of EMT markers in SCC-4 or SCC-15 cells was analyzed by Western blot analysis. β-Actin was used as a loading control. (B) Histogram of Young’s moduli of the non-treated (upper) and TGF-β1-treated SCC-4 cells (bottom, 5 ng/mL for 24 h). (C) The average Young’s moduli determined from the cell centers of non-treated and TGF-β1-treated SCC-4 cells (*** p<0.001). The error bar represents the mean±S.D.
Cancer metastasis involves physical and mechanical two-way interactions between cancer cells and microenvironments.19) Previous nano-mechanical assays suggested that the reduced mechanical stiffness, i.e., elastic moduli could be a diagnostic marker for cancer cells.18) Recent studies also evaluated the potential application of the mechanical stiffness as a clinical diagnostic tool for cancer.41,42) Circulating tumor cells and human biopsied tissues of breast cancer were investigated for this purpose. There were attempts to investigate whether the mechanical stiffness could reflect the degree of oral cancer progression using the AFM indentation techniques or an optical stretcher.33–35) All of these investigations including our results consistently support a gradual decrease in the mechanical stiffness as the OSCCs progress. Thus, the mechanical stiffness could be a promising quantitative marker to diagnose and prognose the progression of oral cancers. However, the measured elastic moduli varied from a few hundred Pa in our study to a few MPa in Lasalvia et al.’s report.34,35) This quantitative discrepancy in elastic moduli could be originated from the fact that we examined the different cell lines from their studies although all the cell lines are OSCCs. In addition, differences in the experimental details of the AFM studies such as variations in the applied trigger forces and the spring constants of the AFM cantilevers could be causes for such observations. For example, Lasalyia et al. applied trigger forces varying from 60 to 120 nN while 0.5 to 1 nN of trigger forces were used in our study34) (Fig. 4A).
The mechanical stiffness of the observed oral cancer cell lines—SCC-4, SCC-9, and SCC-15—(Fig. 4B) resembles that of breast cancer cell lines (MCF-7, MD-MB231) determined from our previous study.29) Interestingly, both studies performed on oral and breast cancer cell lines consistently showed the enhanced mechanical compliance as a hallmark of highly motile or invasive phenotypes. It is highly plausible that such a mechanical softening might allow cancer cells to easily deform and effectively penetrate through the underlying stroma and endothelium, especially in this range of mechanical stiffness (ca. 1000 Pa). However, the opposite correlation—the reinforced mechanical stiffness for motile phenotypes—was observed from prostate and ovarian cancer cells.30–32,43) The elastic moduli of these cancer cells lie in the lower spectrum (< 500 Pa) compared to others and thus the further decrease in the elastic moduli may not be optimal for the effective invasion. This two different correlations could be associated with differences in mechanisms governing the cellular motility—a bleb-associated migration through actomyosin contractility vs. an elongated migration through actin polymerization.44–46) The later mechanism could fit better for the motile behavior of the oral and the breast cancer cells. It can be postulated that the fast turnover of actin polymerization required in the elongated migration mechanism might result in shortened actin filaments and thus mechanical softening in the advanced breast and oral cancer cells.
The EMT as an initial step of the metastatic journey often leads to the treatment failure of OSCCs. The EMT is an orchestrating event accompanied by biochemical as well as mechanical alterations. The EMT-associated biochemical changes include the upregulation of E-cadherin and vimentin, which are often indicators of poor prognosis in cancer patients.47) As shown in Figs. 1C and D, in this study, the upregulation of N-cadherin and vimentin together with the downregulation of E-cadherin supports the EMT progression in SCC-9 and SCC-15 cells similar to the enhanced migratory capacities (Fig. 2). Moreover, we found that mechanical softening of three different OSCCs determined by the AFM indentation technique was exacerbated (Fig. 4B) as the OSCCs acquired the enhanced ability for migration (Fig. 2B). At the extreme end, SCC-15 cells showed two times more migratory capacities and about five times more decrease in the mechanical stiffness compared with SCC-4 cells. This correlation between mechanical stiffness and EMT status among different OSCC cell lines was further confirmed by treating SCC-4 cells with TGF-β1. The expression pattern of EMT markers distinctively differed between TGF-β1-treated and non-treated SCC-4 cells, indicating that the treatment of TGF-β1 induced mesenchymal transition in SCC-4 cells (Fig. 6A). In addition, our AFM measurements revealed that the treatment of TGF-β1 resulted in the mechanical softening (Figs. 6B, C). The decrease in average Young’s moduli of TGF-β1-treated SCC-4 cells was mainly ascribed from the loss of incidences observing Young’s moduli greater than 2 kPa. In other words, the treatment of TGF-β1 make the distribution of elastic moduli more tightly regulated. This observation resembles the transition of Young’s moduli from SCC-4 to SCC-9 cells (Figs. 5A, B). In conclusion, we believe that our data obtained from the TGF-β1 treatment manifestly showed the direct correlation between the mechanical softening and the EMT signatures in OSCCs.
Although vimentin as a major filamentous protein of cytoskeletal components is expected to provide mechanical rigidity to cells, little is known for its mechanical role in cancer progression.48,49) One previous study suggested that the further growth of invadopodia required the entry of vimentin filaments as well as microtubules for the successful transmigration through the basement membrane.50) Rathje et al. also reported that histone deacetylase 6 (HDAC6) induced the structural collapse of vimentin and led to the mechanical reinforcement.40) Consistent with this observation, we also found the perinuclear localization of vimentin and the enhanced mechanical stiffness at SCC-4 cells compared with SCC-9 and SCC-15 cells. In order to be conclusive about the role of vimentin in cells’ structural rigidity, a deformational regime controlled by the trigger forces applied to cells should be considered. Nevertheless, our results suggest that the upregulation of vimentin in cancer cells induces mechanical softening and the enhanced ability for migration unlike other filamentous components in the cytoskeleton. The enhanced polymerization of microtubules as well as actin filaments is known to result in the mechanical reinforcement and the stationary behavior in cancer cells.
In conclusion, our study has reported that OSCC cell lines exhibiting EMT signatures showed a decrease in the mechanical stiffness compared with those without EMT signatures. Here, we suggest that our study combining the AFM-based nano-mechanical and biochemical signaling study focusing on the EMT process could better elucidate the mechanism of OSCC progression and further help us to identify novel therapeutic targets to prevent metastasis in OSCC.
This research was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIP) (2015R1C1A2A01052717 to S. Park) and by the Ministry of Education (2014R1A1A2054979 and NRF-2016R1A6A1A03011325 to C.-H. Jeong).
The authors declare no conflict of interest.