2016 Volume 39 Issue 9 Pages 1555-1558
2016 Volume 39 Issue 9 Pages 1555-1558
Hydrodynamic tail vein injection was considered an in vivo transfection method that yields a higher level of gene expression mainly in the liver. This method has been applied to cancer gene therapy targeting both hepatic and non-hepatic cancers. However, intratumor transgene expression in non-hepatic tumors has not been well studied. In this study, we showed an extended transgene expression of β-galactosidase (LacZ), a nonsecretory protein, in a subcutaneously implanted murine solid tumor following the hydrodynamic injection of plasmid DNA (LacZ pDNA). Our result may indicate that the hydrodynamic injection method is a powerful tool that can be used to gain transgene expression not only in the liver but also in solid tumors.
Hydrodynamic injection is a promising technology that is used to achieve sufficient transgene expression in vivo. This method is characterized by the rapid (5–7 s) injection of a large volume (2 to 3 mL; volume equal to 8–10% of murine body weight) of plasmid DNA (pDNA) solution. A gene transfer method using a large volume of pDNA solution was first reported by Budker et al.1) Since then, two groups improved the method and conceptualized hydrodynamic injection in 1999.2,3) The transgene expression levels gained by hydrodynamic injection usually reached a therapeutic level in the liver.4,5) Such a high transgene expression is considered a preferable feature of hydrodynamic injection; since the liver is the main organ for metabolism, the method has been used in the treatment of congenital metabolic abnormalities6,7) and metabolic syndromes.8,9) Moreover, sufficient transgene expression was observed in other organs, while the expression levels were universally lower than those in the liver.2)
Meanwhile, solid tumors are another important target of transgene expression induced by hydrodynamic injection. Tumor treatments using hydrodynamic tail vein injections were performed as a way to express anticancer secretory proteins such as interferons10) and interleukins.11) In such trials, therapeutic proteins were produced by tumor tissues and/or other organs such as the liver. On the other hand, gene therapy with nonsecretory proteins has never been carried out using hydrodynamic tail vein injection. Unlike secretory proteins, nonsecretory proteins must be expressed in the tumor. Therefore, the potential of cancer gene therapy using nonsecretory proteins is dependent on the extent of gene expression in the tumor tissue.
Concerning intratumor gene expression in hydrodynamic tail vein injections, Tada et al.12) showed that gene expression could be achieved in hepatocellular carcinoma cells in the liver, but the extent of this expression was lower than that found in nontumorous hepatocytes. The liver has a peculiar fenestrated structure in its capillary vessels that is referred to as the sinusoid,13) and this structure is favorable to access by hepatocyte macromolecules in capillaries. The sinusoid in the hepatocellular carcinoma area, however, has fewer fenestrae than that found in nontumorous liver areas.14) Such a difference in the accessibility of pDNA might lead to a different extent of gene expressions between nontumorous hepatocytes and hepatocellular carcinoma areas in the liver. In subcutaneously implanted tumors, leaky blood vessels with enlarged gaps between the endothelial cells allow macromolecule penetration into the tumor tissue, which results in the selective tumor accumulation of macromolecules.15) This has led to the assumption that the increased blood pressure caused by hydrodynamic injection may further enlarge the gaps between blood endothelial cells and consequently enhance the penetration of pDNA into tumor tissue. Based on this assumption, we evaluated the transgene expression in a subcutaneously implanted tumor following hydrodynamic tail vein injection.
We can report that the hydrodynamic injection of pDNA brought a sufficient and broader transgene expression into the solid tumors that were subcutaneously implanted.
pDNA coding LacZ (pCpGfree-LacZ) was purchased from Invivogen (San Diego, CA, U.S.A.). X-Gal (5-bromo-4-chloro-3-indolyl-beta-D-galactoside) was purchased from TaKaRa Bio Inc. (Shiga, Japan). β-Galactosidase was purchased from TOYOBO (Osaka, Japan). Protease Inhibitor Cocktail for Use with Mammalian Cell and Tissue Extracts was purchased from Nacalai Tesque (Kyoto, Japan). All other reagents were of analytical grade.
Animal and Tumor Cell LinesFive-week-old BALB/c male mice were purchased from Japan SLC (Shizuoka, Japan). The mice had free access to water and mouse chow, and were housed under controlled environmental conditions (constant temperature, humidity and a 12 h dark/light cycle). All animal experiments were evaluated and approved by the Animal and Ethics Review Committee of Tokushima University.
The Colon26 murine colorectal carcinoma cell line was purchased from Cell Resource Center for Biomedical Research (Institute of Development, Aging and Cancer, Tohoku University). The Colon26 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) in a 5% CO2/air incubator at 37°C.
Evaluation of the Distribution of Transgene Expression in Tumor and LiverMice were subcutaneously implanted with Colon26 cell suspension (2×106 cells/100 µL DMEM). When tumor volume reached 100 mm3, mice were injected intravenously with pDNA coding LacZ (1, 2, 3, or 4 µg/1, 2, or 2.5 mL saline/mouse). At 13 or 24 h post-injection, the livers and tumors were excised and then embedded in Optimal Cutting Temperature compound (Sakura Finetek Japan, Tokyo, Japan) followed by freezing at −80°C. Frozen samples were cut into 10-µm thick sections using a CM3050S cryostat (Leica Biosystems, Nussloch, Germany). Tissue sections were fixed in 1% glutaraldehyde for 10 min and rinsed three times with phosphate buffered saline (PBS). X-Gal solution (1 mg/mL) was mounted on the sections and incubated at 37°C for 20 h. The sections were rinsed in PBS and examined via bright field microscopy (BZ-9000 microscope, Keyence, Osaka, Japan).
Quantification of β-Galactosidase in the Livers and Tumors of Transfected MiceTumor-bearing mice were prepared as described above. Tumor-bearing mice were injected intravenously with pDNA coding LacZ (2 µg/2 mL saline/mouse). At 13 or 24 h post-injection, the mice were sacrificed and the livers and tumors were excised. Tissue samples were frozen and then homogenized in the lysis buffer (0.25 M Tris, 0.25 v/v% NP-40, 1 v/v% Protease Inhibitor Cocktail, pH 7.4) using a Multi-beads Shocker (Yasui Kikai, Osaka, Japan). Homogenates were subjected to freeze-thaw action for complete lysis of cells, then centrifuged at 12000×g for 5 min at 4°C and the supernatants were used for the following assay. The amount of β-galactosidase was quantified following the previous report with modification.16) Aliquots of the lysates were reacted at 37°C with the assay mixture (0.88 mg/mL 2-nitrophenyl-β-D-galactopyranoside (ONPG), 1 mM MgCl2 in 0.1 M sodium phosphate buffer). The production of enzymatic product is evaluated by a spectrophotometer at 405 nm. The amount of β-galactosidase was determined by standard curve method using with commercial β-galactosidase (TOYOBO, Osaka, Japan). Data for the quantity of β-galactosidase were represented as the units of β-galactosidase per g of wet weight of tissue (μU/g tissue).
Statistical AnalysisAll values were determined as the mean±standard deviation (S.D.). Statistical analysis was accomplished via a two-tailed unpaired Student’s t-test using GraphPad InStat software (GraphPad Software, CA, U.S.A.). The level of significance was set at p<0.05.
pDNA-coding LacZ (2 µg pDNA/2 mL saline/mouse) was administered into tumor-bearing mice via hydrodynamic injection. Tumors and livers were collected 13 h after administration. Then, the distribution and magnitude of LacZ gene expression was evaluated using blue dye, and was based on the production of X-gal cleaved by the LacZ gene, β-galactosidase. As shown in Fig. 1A, LacZ was expressed partially but strongly in the liver. The blue spots were 100–1000 µm in diameter. On the other hand, in the tumor, LacZ expression was much weaker but much extended (Fig. 1B). The blue spots (LacZ expression) exhibited a particulate pattern (ca. 10 µm in diameter) in the whole tumor tissue section. The magnitude of gene expression in the tumor was lower than that in the liver by three-orders of magnitude (Fig. 1C). From 13 to 24 h after injection, gene expression in the tumor was increased by similar rate with that in the liver. These findings indicate that the hydrodynamic injection of pDNA can achieve a transgene expression not only in liver but also in tumor tissue.
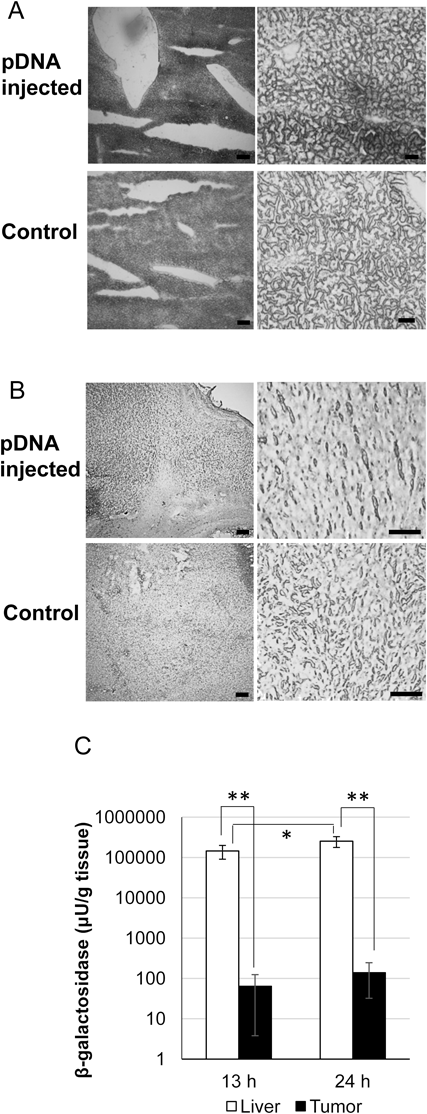
(A) Lac-Z gene expression in a liver section. (B) Lac-Z gene expression in a tumor section. Tumor-bearing mice (n=3) received pDNA-expressing LacZ (2 µg pDNA/2 mL saline/mouse). At 13 h post-injection, the livers and the tumors were excised. As a control, those of non-treated tumor-bearing mice were also excised. Cryosections of the livers and tumors were prepared and stained with X-gal solution. The images of sections were obtained using a BZ-9000 microscope with a 4× objective lens for low-power field images (left columns) and 20× (for liver) and 40× (for tumor) objective lenses for high-power field images (right columns). Bars=200 µm (left columns), 50 µm (right columns). (C) The quantification of Lac-Z gene expression. Tumor-bearing mice (n=5 for each timepoint) received pDNA-expressing LacZ (2 µg pDNA/2 mL saline/mouse). At 13 h or 24 h post-injection, the livers and the tumors were excised. Tissue lysates were prepared and then reacted with ONPG. The quantity of β-galactosidase was estimated from the absorbance of enzymatic product at 405 nm and represented as the units of β-galactosidase per g of wet weight of tissue (μU/g tissue). Statistical differences were evaluated by a two-tailed unpaired Student’s t-test (* p<0.05,** p<0.01).
In the hydrodynamic injection, the effect of pDNA doses (1, 2, 3, 4 µg pDNA) and the volumes (1, 2, 2.5 mL) of pDNA solution on transgene expression in tumor tissue were studied. As shown in Fig. 2A, the strength of the blue stain tended to correspond to the pDNA dose. On the other hand, the density was not elevated by increasing the injection volumes of pDNA solution (Fig. 2B).

(A) Dose dependent transgene expression in tumor. (B) No volume dependent transgene expression in tumor. Tumor-bearing mice were injected intravenously with pCpGfree-LacZ (1, 2, 3, 4 µg pDNA/2 mL saline/mouse or 4 µg pDNA/1, 2, 2.5 mL saline/mouse). At 24 h post-injection, the tumors were excised. Cryosections of tumors were prepared and stained by X-gal solution. The images of sections were obtained using BZ-9000 microscope with 4× objective lens. Bars=2000 µm.
In the present study, we showed that the hydrodynamic tail vein injection method is applicable for gene delivery to a subcutaneous tumor. Of interest, the distribution pattern of transgene expression in the tumor seemed to be different from that in the liver. The blue spots indicating LacZ-gene expression in the tumor tissue were small (ca. 10 µm) and pale. In contrast, such spots in the liver were much larger (100–1000 µm) and darker. This difference might be accounted for by the diameter and structure of the blood vessels in liver compared with that in a subcutaneous tumor. The liver has wider blood vessels such as the portal vein, the hepatic artery, and the hepatic vein. Injected pDNA from the tail vein would flow into the hepatic vein in a retrograded manner with high pressure and cause extravasation in the fenestrated microvessels, namely the sinusoids. Therefore, the pDNA that penetrated the multiple capillary vessels might have produced extensive transgene products and consequently a large transgene expression area (Fig. 1A). On the other hand, in contrast to the clustered distribution of the hepatic sinusoid,17) tumor vessels would not form such clusters because tumor angiogenesis is a disorderly process. Accordingly, pDNA administered by hydrodynamic tail vein injection was supposed to leak out from the tumor vessels with a dispersed distribution and to form small, multiple spots of transgene expression in the tumor tissue (Fig. 1B). The amount of LacZ in both tumor and liver was increased with increasing the time after injection (Fig. 1C). At a glance, the magnitude of gene expression was also increased. But, because LacZ was stable protein, the increased LacZ amount in the tissue might reflect accumulation of gene product. Further investigation using pDNA coding the reporter protein with shorter half-life would be required for evaluating the precise time dependency of gene expression in the tumor tissue.
In the tumor tissue, dose dependent LacZ gene expression was observed (Fig. 2A), but volume dependent one was not observed (Fig. 2B). Liu et al.2) have reported that transgene expression in liver by hydrodynamic injection occurred in a dose and volume dependent manner. Increasing injection volume with fixed pDNA dose leads the decrease of pDNA concentration in the injection solution. The decreased pDNA concentration may affect the magnitude of gene expression in the tumor more strongly than injection volume.
In conclusion, we showed that hydrodynamic injection of naked pDNA is applicable for gene transfer into subcutaneous tumors. This technique may enable broad transgene expression in tumor tissue and might contribute to the functional evaluation of nonsecretory therapeutic proteins and gene carriers.
The authors are grateful to Mr. James L. McDonald for his helpful advice in developing the English manuscript. This study was supported by a research program for the development of intelligent Tokushima artificial exosome (iTEX) from Tokushima University.
The authors declare no conflict of interest.