2017 Volume 40 Issue 1 Pages 1-10
2017 Volume 40 Issue 1 Pages 1-10
The liposome, a closed phospholipid bilayered vesicular system, has received considerable attention as a pharmaceutical carrier of great potential over the past 30 years. The ability of liposomes to encapsulate both hydrophilic and hydrophobic drugs, coupled with their biocompatibility and biodegradability, make liposomes attractive vehicles in the field of drug delivery. In addition, great technical advances such as remote drug loading, triggered release liposomes, ligand-targeted liposomes, liposomes containing combinations of drugs, and so on, have led to the widespread use of liposomes in diverse areas as delivery vehicles for anti-cancer, bio-active molecules, diagnostics, and therapeutic agents. In this review, we summarize design optimization of liposomal systems and invaluable applications of liposomes as effective delivery systems.
Since the pioneering observation of Alec Bangham in 19641) that phospholipids in aqueous systems can form closed bilayered structures, liposomes have emerged as one of the most promising tools for drug targeting in medical fields. The inherent advantages of liposomes, such as biocompatibility, low toxicity, high loading capacity, and controllable release kinetics,2–4) have inspired their efficient use as drug carriers and triggered the first approval of a liposomal formulation for the treatment of fungal infections: Ambisome®, a conventional liposomes encapsulating the antifungal agent, amphotericin B.5) However, despite this success, the in vivo fate of such conventional liposomes was dismal. Conventional liposomes tend to fuse and/or aggregate with each other resulting in immature release of liposomal payload over time.6,7) In addition, conventional liposomes underwent rapid systemic clearance via their uptake by the cells of mononuclear phagocyte system (MPS).8,9) To overcome these limitations, surface-modification strategies were adopted by coating the surface of conventional liposomes with inert, biocompatible hydrophilic polymers, such as polyethylene glycol (PEG). Such hydrophilic polymer confers steric stabilization to the liposomal surface via formation of a protective layer over the liposome surface and slows down liposome recognition by opsonins, and therefore, subsequent clearance of liposomes.10,11) As a result, stealth technology, PEGylation, has led to the development of several clinically approved liposomal formulations such as Doxil®, Caelyx®, and Myocet® used for the treatment of cancers.12,13)
Nonetheless, despite the improved pharmacokinetics of PEGylated liposomes, the lack of target selectivity substantially restricted the therapeutic potential of PEGylated liposomes in many clinical settings. Accordingly, several engineering strategies have been applied to improve the in vivo performance of liposomes. These strategies include either the attachment of site-directed surface ligands, such as antibodies (immunoliposomes),14,15) positive charge (cationic liposomes),16,17) or a peptide (peptide-targeted liposomes),18,19) or exploiting the inherent physiological conditions in the target tissue, such as elevated temperature or alteration in pH (Fig. 1) so as to produce stimuli-responsive liposomes such as thermo-sensitive liposomes2,20,21) and pH-sensitive liposomes.22,23)

(A) Classical liposome encapsulating lipid soluble drugs; (B) classical liposome encapsulating aqueous soluble drugs; (C) sterically stabilized liposomes; (D) ligand-targeted liposome containing an aqueous soluble drug. Figure modified from: Abu Lila A, Ishida T, Allen T. “Liposomal Nanomedicine, Handbook of Nanobiomedical Research (Torchilin V, ed.). World Scientific Publishing, 2014.123)
Several recent reports have discussed specific aspects of liposomes regarding novel structural formulations or current clinical progress. In this review, we highlight some inherent liposomal problems facing their optimized design. We also place special focus on recent applications of liposomes as efficient delivery systems.
Drug loading into nanoparticles, including liposomes, is known to increase the therapeutic ratio of the entrapped drug by permitting selective delivery of adequate concentrations of the entrapped drug to the site of action while restraining its delivery to non-target (normal) tissues.24,25) However, the therapeutic impact of many liposomal formulations was compromised in various experimental/clinical settings by, at least in part, low drug entrapment efficiency. The commercial impact of liposomes is strengthened by the invention of active “remote” drug loading methods, allowing the encapsulation of several weak base or weak acid drugs with very high drug-to-lipid ratios (encapsulation efficiency up to 90%).26) In the active loading method, the pH or chemical composition of the internal aqueous compartment of the liposomes is manipulated to allow efficient retention of drugs within the liposomes. This method was widely applied for the efficient entrapment of drugs such as doxorubicin, daunorubicin, and vincristine.27)
Although the above strategy has proven advantageous in certain circumstances, active loading is not considered a universal method with many drugs that are highly hydrophobic or lack an ionizable group, such as paclitaxel or ciprofloxacin, which cannot be remotely loaded into liposomes.27–29) Nonetheless, Sur et al.30) have recently reported an innovative method to allow the active loading of hydrophobic chemotherapeutics devoid of ionizable groups. They employed modified cyclodextrins with ionizable groups on their surfaces. The “pockets” of these cyclodextrins can encapsulate hydrophobic drugs and ferry them across the bilayer membrane of conventional liposomes using simple pH gradients. They emphasized that the drug : lipid ratios achieved through this unique approach was >1000-fold higher than those commonly achieved through passive loading.31–33)
2.2. Control of Drug Release RateIt is well recognized that the entrapped drug within liposomes is not bioavailable until it is released.34) Therefore optimizing the release rate of liposomal payload is considered a crucial determinant for the overall therapeutic efficacy of liposomal systems. As a general rule, liposomes should grant the delivery of adequate concentration of bioavailable drug within the target tissue, at an appropriate rate, for a sufficient period of time, while retaining the drug during transit to the target site (i.e., no premature release).35–37) Kim et al.38) have reported that despite the efficient intratumor delivery of stealth liposomal formulation of cisplatin in mouse tumor model, the liposomes failed to exert any therapeutic potential. They attributed such conflicting results to the failure of liposomes to release a minimum cytotoxic concentration of cisplatin at the tumor tissue. We observed similar results after intrapleural administration of cholesterol-containing liposomes loaded with pemetrexed in orthotopic malignant mesotheliomal tumor model.39)
Drug release from liposomes was demonstrated influenced by liposomal membrane composition and the physicochemical properties of the encapsulated drug.8,40,41) Inclusion of cholesterol into the liposomal membrane, which has a membrane rigidizing effect,42,43) and switching from a fluid phase phospholipid bilayer to a solid phase bilayer42,44) were shown to reduce the release of drugs from liposomes. Drugs with extremely low octanol/buffer partition coefficients exhibited prolonged liposomal retention whereas molecules with partition coefficients ranging from −0.3 to 1.7 were, in contrast, rapidly released.45) Interestingly, the effect of drug-to-lipid ratio on drug release is also dependent on the specific drug entrapped. In the case of doxorubicin, a higher drug-to-lipid ratio resulted in decreased drug retention half-life.46) In contrast, vincristine and irinotecan were retained longer in liposomes with higher drug-to-lipid ratios; a 10-fold increase in release half-life was observed as drug-to-lipid ratio was increased from 0.05 to 0.6 (w/w).47)
2.3. Manipulation of the in Vivo Fate of LiposomesExcept for the treatment of diseases where there was an MPS involvement, e.g., leishmaniasis,48) the rapid liposomal uptake by the macrophages and their consequent removal from circulation represented a major challenge against the potential use of “classical” liposomes as drug delivery systems.49,50) Accordingly, many attempts have been devoted to enhance their pharmacokinetic characteristics following systemic administration. Initially, manipulation of the physicochemical properties of liposomes, such as size, fluidity, net surface charge, and packing of the lipid bilayers, has been found to affect not only liposomal physical stability but also their uptake by cells of the MPS.51,52) Charged liposomes and/or large-size liposomes are cleared from the systemic circulation more rapidly than neutral and/or small-size liposomes.53,54) In addition, the use of saturated phospholipids or the incorporation of cholesterol, which increases the packing of phospholipids in the lipid bilayer, reduces liposomal uptake by cells of the MPS.8,55) However, this approach cannot fully overcome the binding of classical liposomes with serum components and only slightly decreased MPS uptake of liposomes. An early alternative approach was to administer large pre-doses of “empty” classical liposomes to saturate the phagocytic uptake capacity of cells of the MPS.56,57) Thereafter, a more fascinating approach to enhance the pharmacokinetics of liposome was via liposomal surface decoration with inert, biocompatible hydrophilic polymers, such as ganglioside GM1, phosphatidylinositol, or lipid-conjugated PEG.58–60) These polymers act by forming a hydrophilic protective layer over the liposome surface that sterically hinders the attachment of serum opsonins to the liposomal surface and accordingly slows down the systemic clearance of liposomes.37,60) By reducing MPS uptake, longer-circulating liposomes have enhanced opportunity to accumulate inside the target tissue via what is called passive targeting. This phenomenon is highly manifested in solid tumors where the leaky nature of tumor vasculature triggers the preferential accumulation of liposomes into the tumor tissue via a process known as enhanced permeation and retention (EPR) effect.61) As a result of this success, long-circulating liposomes are currently adopted in clinical practice.2,62)
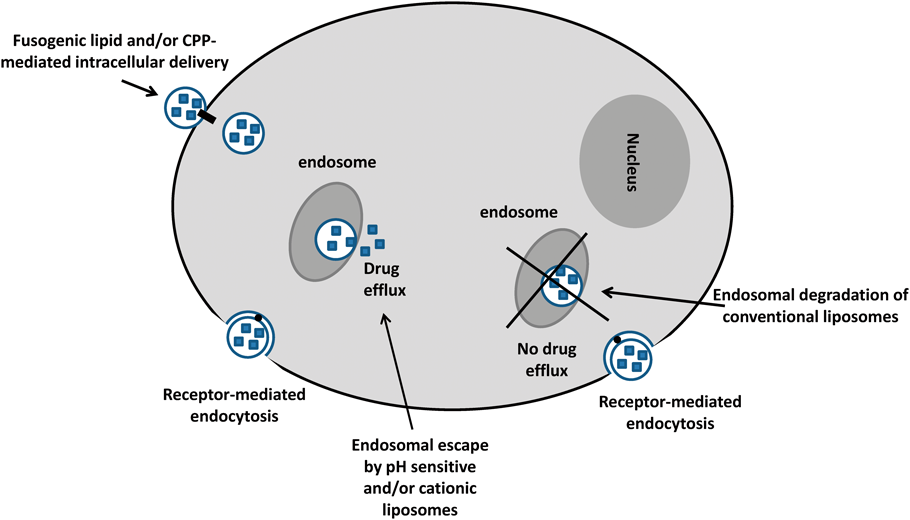
2.4. Enhancing Intracellular Delivery of Liposomal PayloadDespite the widespread use of liposomes as attractive delivery vehicles for drugs and/or biomolecules, their clinical efficiency was severely compromised by the inefficient intracellular delivery of encapsulated payload. The lipophilic nature of the biological membranes restricts direct entrance of liposomes into target cells. Instead, liposomes are engulfed into cells via phagocytosis and/or endocytosis.63) Nonetheless, receptor-mediated endocytosis of liposomes results in their lysosomal delivery, where they become vulnerable to degradation by the acidic and enzyme-rich environment of the endosomes and lysosomes.34,64) Recently, much attention has been devoted to verifying different strategies to bypass the endocytic pathway (endosomal escape) and, thereby, enhancing the therapeutic efficacy of encapsulated drug. The use of pH-sensitive peptides or cationic lipids is considered an efficient strategy to enhance the cytoplasmic delivery of liposomal cargo by disrupting the endosome/lysosome membrane following liposomal uptake by phagocytosis and/or endocytosis.64–67) Alternatively, incorporation of fusogenic lipids into liposomes and/or the use of cell-penetrating proteins or peptides (CPPs), proteins and peptides that can translocate through the cellular membranes, reportedly may enhance cytoplasmic delivery of liposomal cargo via an endocytosis-independent manner.68–70) However, these non-endocytotic pathways are less prominent than those using endocytosis (Fig. 2).
Liposomes have demonstrated a wide range of applications in clinical settings. These applications ranged from therapeutic and diagnostic to, most recently, theranostic applications (Fig. 3). In this section, we summarize the potential application of liposomes in each field separately.
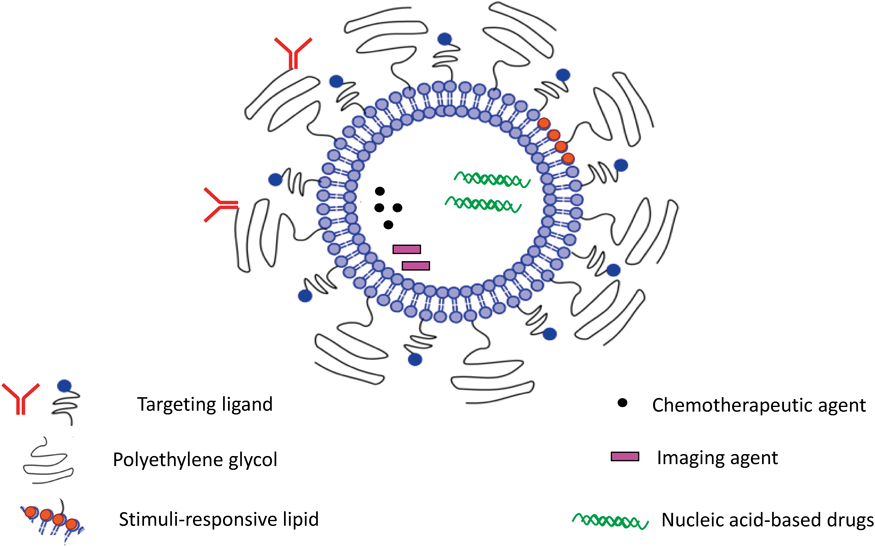
Figure modified from: Abu Lila A, Ishida T, Allen T. “Liposomal Nanomedicine, Handbook of Nanobiomedical Research (Torchilin V, ed.). World Scientific Publishing, 2014.123)
A mounting body of literature has defined the viability of formulating a wide range of drugs in liposomes taking advantage of improved therapeutic efficacy and/or reduced systemic toxicity of the encapsulated drug compared with the free counterpart. Generally, alteration of the pharmacokinetics of liposomal drugs, via encapsulation, can lead to prolonged blood circulation characteristics, enhanced drug bioavailability, and/or preferential accumulation in disease sites.
3.1.1. Small Molecule TherapeuticsDelivery of small molecule therapeutics, particularly chemotherapeutic agents, was one of the first clinical applications of liposomal delivery systems. Conventional chemotherapeutic agents may indiscriminately kill not only diseased cells but also rapidly growing healthy cells, leading to severe side effects on tissues such as blood cells and hair follicles and on cells lining the intestinal mucosa,71) and inflicts practical limits on the drug dose and dosing frequency. Exploiting the patho-physiological conditions of tumor tissue, such as leaky tumor vasculature, along with drug encapsulation within a nanocarrier represented an invaluable strategy for combating cancer. Classical liposomes or PEGylated liposomes use a passive targeting strategy, mediated by the EPR effect, preferentially to accumulate the encapsulated drug into the tumor tissue and, thereby, enhance its therapeutic efficacy while minimizing its side effects.61) A brilliant example is the clinical use of Doxil®, a PEGylated liposomal formulation of doxorubicin (DXR), an anthracycline anticancer agent active against a wide variety of solid tumors.72,73) Liposomal encapsulation of doxorubicin was reported to enhance the therapeutic efficacy of the encapsulated drug, in tandem with reducing the risk of anthracycline-induced cardiotoxicity, which potentially limits the clinical use of the free drug.74) After the approval of Doxil® in 1995, a number of cytotoxic agent-containing liposomes have emerged for clinical use whereas more liposomal formulations of anticancer agents are currently in various stages of clinical trials.
Despite the aforementioned advances achieved by passive targeting-mediated delivery of liposomal anticancer drugs, many reports have emphasized the failure of such targeting strategy in ensuring the adequate delivery of a minimum therapeutic concentration of encapsulated drug within tumor tissue, resulting in treatment failure. Alternatively, an active “ligand-mediated” targeting approach has been extensively investigated for its ability to improve intracellular delivery of encapsulated drug within tumor tissue. A variety of molecules, including peptides, antibodies, proteins, charged molecules, and some low-molecular weight ligands, such as folate, and nucleic acid-based aptamers, have been studied in conjunction with liposomes for enhanced anticancer treatment.75–78)
Enhanced tumor targeting can also be attained via exploiting specific features in the tumor microenvironment such as lowered extracellular pH79) and an altered pattern of extracellular proteins.80) For example, Zhu et al.80) exploited elevated levels of matrix metalloprotease 2 in the tumor microenvironment to improve cancer cell-specific delivery of loaded drugs via conjugating the anti-nucleosome monoclonal antibody (mAb 2C5) to PEGylated liposomes. Another strategy is to use external stimuli, such as a magnetic field,81,82) altered temperature,81,83) ultrasound,84) and light85) to improve delivery of encapsulated anticancer agents to tumors.
In addition to cancer chemotherapy, liposomal delivery systems have been used for the treatment of other diseases. For example, encapsulation of the antifungal drug, amphotericin B, within conventional liposomes has been reported to increase the overall therapeutic efficacy while minimizing severe renal and neuronal toxicities.86) This led to the approval of this agent under the name of AmBisome® for the treatment of severe systemic fungal infections in immunocompromised patients.87)
The inherent ability of cells of the MPS, mainly macrophages of the liver and spleen, to engulf conventional liposomes has also been exploited for the delivery of anti-microbial drugs to cells of the MPS, which are considered the main reservoirs of parasites.2,34) Demicheli and colleagues have investigated the efficacy of liposomal encapsulation of the antimonial drug, meglumine antimoniate, in hamsters experimentally infected with Leishmania chagasi. They revealed that a significant reduction of liver parasite burden was observed in animals treated with the liposomal formulation. In contrast, free meglumine antimoniate was inefficient when administered at a comparable dose of antimony.88)
3.1.2. Gene TherapySoon after the approval of liposomes as delivery vehicles for small molecule therapeutics, liposomes were investigated for their ability to deliver macromolecules such as nucleic acid-based therapeutics (plasmid DNA (pDNA), antisense oligonucleotides (asODNs), and small interfering RNA (siRNA)) to disease sites. Nucleic acid-based materials are high-molecular weight, hydrophilic, highly charged molecules that cannot cross cell membranes by passive diffusion. In addition, rapid enzymatic degradation and systemic clearance, low selectivity for the desired tissue, and poor cellular uptake of nucleic acid-based materials significantly limit their clinical application. Accordingly, liposomes have been challenged for their ability to deliver nucleic acids-based therapeutics to target cells/tissues. For such purpose, charge-imparting lipids, such as 1,2-bis(oleoyloxy)-3-(trimethylammonio)propane (DOTAP) and 3β[N′,N′-dimethylamino-ethane]-carbomoyl] cholesterol (DC-CHOL), were incorporated into the membrane of liposomes to impart them with a net positive charge. These positively charged “cationic” liposomes interact physically with the negatively charged nucleic acid-based materials to form a complex structure known as “lipoplex.” These lipoplexes are thought to enter the cell through fusion with the plasma membrane, and can promote release of nucleic acids from endosomal membranes following internalization.89) Nonetheless, the potential clinical use of “cationic” liposomes is limited by their instability, rapid systemic clearance, toxicity, and induction of immunostimulatory responses.
Alternatively, further to maximize the potential use of liposome-based systems for gene therapy, a number of techniques were devoted to develop cationic lipid-containing liposomes that efficiently entrap nucleic acids in their interior, but which have a net neutral or anionic surface charge. These include coated cationic liposomes (CCL),90) lipidic nanoparticles (LNP),91) stable nucleic acid lipid particles (SNALP),92) and liposome-polycation-hyaluronic acid particles (LPD).93) In addition, the cell selectivity of the surface neutral liposome-nucleic acid complex can be improved by the application of cell-specific targeting technology.94) Pastorino et al.95) have developed long-circulating cationic liposomes (CCL) entrapping c-myb asODNs and targeted against the ganglioside GD2 significantly to suppress tumor growth and metastases in murine models of melanoma96) and neuroblastoma.95)
A novel lipid bilayer containing a mixture of cationic and fusogenic lipids coated with diffusible PEG, known as SNALP, has been designed to protect siRNAs from serum nucleases and allow cellular endosomal uptake and subsequent cytoplasmic release of siRNAs. Wilner and Levy97) have recently described a method for using aptamers to deliver SNALP encapsulating siRNA to cells in vitro. Using such SNALP, selective targeted delivery along with efficient siRNA-mediated gene knockdown can be realized.
Clinically, LNP have been investigated for their potential to deliver RNA interference (RNAi) molecules to target sites. In one clinical trial, LNP siRNA system was used to silence expression of PCSK9, a gene responsible for modulation of low-density lipoprotein cholesterol (LDL-C) levels in the circulation. This LNP siRNA system was confirmed significantly to reduce LDL-C levels without exerting toxic side effects. In another trial, LNP siRNA system targeting transthyretin (TTR), currently investigated for the treatment of TTR-induced amyloidosis, was found to induce a dramatic reduction of TTR levels in blood.34)
It is worth noting that gene therapy can also be combined with small molecule therapy to achieve enhanced efficacy. Saad et al.98) have employed cationic liposomes for co-delivery of DXR and siRNA targeting multi-drug resistance (MDR) protein to enhance anticancer efficacy of DXR in lung cancer cells. Later, Peng et al.99) evaluated the efficiency of a novel thermosensitive magnetic liposome as a vehicle for the co-delivery of both DXR and SATB1 short hairpin RNA (shRNA) to gastric cancer cells. They demonstrated that DXR and SATB1 shRNA can be delivered into human gastric adenocarcinoma MKN-28 cells with high gene transfection and drug delivery efficiency resulting in enhanced growth-inhibitory activity against gastric cancer cells in vitro and in vivo, compared with single delivery.
3.1.3. ImmunotherapyTo date, liposomes have been demonstrated effective immunological adjuvants for protein and peptide antigens.2) They are capable of eliciting both humoral and cellular immune responses for a broad spectrum of infectious diseases and cancers, without causing granulomas at the injection site or producing any hypersensitivity reactions.100) Liposomes with encapsulated protein or peptide antigen are phagocytosed by macrophages and eventually accumulated in lysosomes. Once in the lysosomes, degraded peptides are presented to the major histocompatibility complex class II (MHCII) on the macrophage surface, resulting in the stimulation of specific T-helper cells and specific B cells leading to subsequent secretion of antibodies.101)
Structural modification of liposomes, such as the formulation of pH-sensitive liposomes, endows liposomes with additional advantages over traditional adjuvants by permitting the escape of the peptide antigen from endosomes into the cytoplasm,102) and thus allows the association of antigen with the MHC-I complex, which induces a cytotoxic T-lymphocyte (CTL) response. Such stimulatory effects were not observed with traditional adjuvants such as Freund’s adjuvant.2)
In addition to encapsulation, direct surface modification of liposomes with antigen can also elicit an immunologic response. Guan et al.103) reported that synthetic human mucin 1 peptide, a candidate for therapeutic cancer vaccines, could elicit a potent antigen-specific T-cell response when being either encapsulated or attached to the surface of liposomes.
Interestingly, lipid constituents of liposomal vesicles can be tailored to achieve particular immunogenic responses. Manipulation of surface charge density in cationic liposomes was reported to regulate immune response. Ma et al.104) investigated the immunogenic response towards ovalbumin after altering the overall charge of liposome surface by changing the ratio of cationic DOTAP to neutral 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC). They confirmed that increasing surface charge density potently enhanced dendritic cell maturation, reactive oxygen species (ROS) generation, antigen uptake, and production of OVA-specific immunoglobulin G2a (IgG2a) and interferon-γ (IFN-γ).
Epaxal®, Inflexal®, and Mosquirix® are clinically approved liposome-based vaccine products, classified as virosomes. Virosomes are liposomes comprising reconstituted viral membranes supplemented with phosphatidylcholine. Epaxal®, a hepatitis A virus vaccine, is based on an inactivated hepatitis A antigen incorporated into the virosomes. Inflexal®, an influenza virus vaccine, is based on hemagglutinin and neuraminidase from inactivated influenza incorporated into virosomes with lecithin.105) Mosquirix®, a malaria vaccine, is based on antigens containing epitopes from the circumsporozoite protein of the Plasmodium falciparum malaria parasite genetically fused to hapatitis B antigen (HBsAg).106) All these vaccines are safe, well tolerated, and generate effective immune responses.
Recently, liposomes have gained much attention as vehicles in nucleic acid vaccines, rather than carriers for attenuated bacterial antigens or protein or peptide vaccines, taking advantage of the higher capacity of liposomes to deliver the encapsulated genetic material, such as pDNA, mRNA, or siRNA, to specific targets. Ribeiro et al.107) used liposomes to deliver pDNA encoding heat shock protein 65 (hsp65) to treat the pulmonary fungal infection paracoccidioidomycosis. They confirmed that liposomal vaccine could modulate a protective immune response and reduced pulmonary fungal burden. Li et al.108) also investigated the potency of liposome encapsulating double strand RNA (LE-PolyICLC) against influenza. They reported that intranasal LE-PolyICLC inhibited virus replication, reduced viral titers, and attenuated pulmonary fibrosis.
3.2. Diagnostic Applications of LiposomesEarly detection of diseases, including cancer, is a crucial determinant of clinical outcomes. As a result, a broad area of research has focused on the development of new diagnostic agents/techniques and optimizing the action of existing ones. Generally, diagnostic/imaging agents are necessary to achieve a sufficiently intense signal from the area of interest so as to differentiate certain structures from surrounding tissues, regardless of the modality used. Consequently, to facilitate the accumulation of imaging “contrast” agent in the required zone, various particulate systems have been investigated as carriers for contrast agents. Among these carriers, liposomes have drawn special attention because of their easily manipulated physicochemical properties and pharmacokinetics. In addition, the multifunctional character of liposomes has permitted the loading of a variety of imaging agents, and the inclusion of specific ligands for efficient targeting to the desired tissue.109) Accordingly, liposomes have been used as carriers for many widely used imaging techniques including magnetic resonance imaging (MRI), ultrasound, fluorescence, and nuclear imaging applications. In this section, we represent some elegant examples of the deployment of liposomes as a diagnostic tool in different imaging modalities.
Fluorescence imaging is one of the most commonly used diagnostic techniques that allows the visualization of gene expression, biomolecule location, and enzyme activity in living cells. Liposomes with improved pharmacokinetics can help deliver fluorescence imaging agents to the target area. Al-Jamal et al.110) designed a hybrid nanoparticle by encapsulating PEG-coated quantum dots (QDs) inside the aqueous phase of DOPC-based liposomes for cancer imaging. These hybrid nanoparticles not only preserve the fluorescence properties of the unmodified QDs but also exert a higher targeting potential to tumor tissues. In addition, in tumor model experiments, these nanoparticles showed enhanced tumor penetration and retention properties in both tumor spheroids and subcutaneous solid tumors, raising their diagnostic potential compared with unmodified QDs.
MRI is a noninvasive medical imaging technique that uses a magnetic field and pulses of radio wave energy to visualize organs and other structures inside the body. A crucial factor to improve the functionality of MRI is the use of paramagnetic contrast agents to probe the tissue/organ under investigation. The ability of liposomes containing MRI contrast agents to circulate in the blood circulation for prolonged times and to deliver these probes in the target site maximized liposomal use in MRI. Tagami et al.111) developed a multifunctional thermosensitive liposomal formulation that enhanced DXR targeting to a locally heated tumor, and by coupling with noninvasive MRI contrast agent (gadopentetate dimeglumine (Gd-DTPA)), the release pattern and the in vivo antitumor efficacy of encapsulated drug, DXR, in the EMT-6 tumor model was successfully traced. Bankiewicz and colleagues112) have also emphasized the use of paramagnetic gadolinium liposomes in direct visualization of the tissue distribution of drugs infused by convection-enhanced delivery to brain tumors via real-time MRI monitoring of liposomes containing gadolinium. Similarly, MRI using pH-sensitive contrast liposomes was reported to enable visualization of pathological areas with decreased pH values.113)
Computerized tomography (CT) is an imaging procedure that uses special X-ray equipment to create detailed pictures of areas inside the body. CT contrast agents can be included in the inner aqueous core of liposomes or incorporated into the liposome membrane. For example, iopromide has been incorporated into plain114) and PEGylated liposomes115) and demonstrated favorable biodistribution and imaging potential in rats and rabbits. Encapsulation of the iodinated contrast agent, iohexol, in the inner aqueous compartment of a ligand-targeted liposome “immunoliposomes” has been reported to enhance the diagnostic potential of encapsulated iodinated contrast agent against atheromatous plaques in activated human coronary artery endothelial cells (HCAEC) via selectively targeting the surface of activated HCAEC with high specificity, compared with free contrast agents.109)
Sonography (ultrasound imaging) is another widely used noninvasive diagnostic imaging technique that is based on the application of ultrasound and measuring the echoes caused by tissues at different reflection angles. Liposomes containing gas bubbles, which are efficient reflectors of sound, can serve as ultrasound imaging probes. Liposomes for sonography are prepared by entrapping gas bubbles into the liposome, or by forming the bubble directly inside the liposome via a chemical reaction, such as bicarbonate hydrolysis yielding carbon dioxide. Gas bubbles entrapped inside the phospholipid membrane showed good efficacy and low toxicity of these contrast agents in animal models.2)
3.3. Theranostic Applications of LiposomesTheranostics is emerging as a promising therapeutic paradigm. It describes the co-delivery of therapeutic and imaging agents in a single formulation; consequently, theranostic-based strategies may be beneficial in the selection of therapy, designing dosage regimen, monitoring of objective response, and planning of follow-up therapy based on the specific molecular characteristics of a disease.116,117) Liposomes are currently considered one of the attractive platforms for theranostic nanomedicine due to their high capacity to ferry cargo and the flexible encapsulation capabilities of both imaging and therapeutic agents.118)
Kaul et al.119) have recently developed a folate-targeted PEGylated liposomal formulation, encapsulating the anti-tuberculosis (anti-TB) drug rifampicin, for in vivo imaging of mycobacterial infections. In vivo scintigraphic studies in a murine model of TB infection showed higher uptake at infected lesions at 2 h post-injection. Blocking imaging experiments showed minimized non-selective uptake, which confirms specific targeting. In addition, therapeutic experiments confirmed the efficient liposomal delivery of the anti-TB drugs in the murine model of infection.
Recently, theranostic nanomedicine, using liposomal nanocarriers, has been extensively investigated in cancer. Lozano et al.120) developed an antibody-targeted PEGylated liposome “immunoliposome” encapsulating the dye indocyanine green (ICG) and the anticancer drug DXR to visualize noninvasively the tumor accumulation of these immunoliposomes over time in a murine breast cancer mouse model. They revealed that such theranostic liposome demonstrated ability to combine tumor-specific targeted therapy with diagnosis using multispectral optoacoustic tomography (MSOT). Similarly, Muthu and colleagues121) used transferrin-conjugated liposomes loaded with docetaxel and QDs for imaging and therapy of brain cancer. The in vivo results indicated that transferrin-conjugated theranostic liposomes provided an improved and prolonged brain targeting of docetaxel and QDs in comparison with non-targeted preparations, granting them with a potential application in brain theranostics. Ren et al.122) designed a multifunctional liposome encapsulating paclitaxel and gadoterate meglumine (Gd-DOTA) (an MRI probe) for targeted tandem chemotherapy and therapeutic response monitoring. The surface of such liposomes was decorated with RGD peptide and fluorophore. Such targeted liposome was able selectively to deliver paclitaxel and Gd-DOTA to tumor tissues in a mouse xenograft model. This was confirmed by fluorescence images, indicating that targeted liposomes could maximize the therapeutic potential of the encapsulated payload, anticancer drugs and contrast agents, while limiting related off-target side effects.
The development of liposomes as carriers for therapeutic molecules is an ever-growing research area. The possibility of manipulating the inherent characteristics of these nanocarriers makes them versatile carriers for a wide range of materials (drugs, proteins, peptides, nucleic acids, and so on) and widens their potential use in many clinical settings. Furthermore, the ability of liposomes to co-encapsulate both therapeutic and diagnostic agents paves the way for a novel application of liposomal delivery systems as theranostic platforms. However, a rational design approach to achieve therapeutic objectives might represent the rate-determining step in the development of more sophisticated lipid-based therapeutics in the future.
This work was supported in part by a Grant-in-Aid for Scientific Research (B) (15H04639), the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The authors declare no conflict of interest.