2017 Volume 40 Issue 10 Pages 1646-1653
2017 Volume 40 Issue 10 Pages 1646-1653
The cross-linking of elastin by lysyl oxidase (LOX) family members is essential for the integrity and elasticity of elastic fibers, which play an important role in the characteristic resilience of various tissues. However, the temporal sequence of oxidation by LOX during elastic fiber formation is still incompletely understood. Here, we demonstrate that the cross-linking of tropoelastin molecules by LOX occurs concurrent with elastin deposition. Our data show that LOX deficiency or the inhibition of LOX enzyme activity leads to the loss of elastin deposition in skin fibroblast. Moreover, overexpression of LOX promotes the deposition and alignment of tropoelastin, whereas the addition of recombinant active-form of LOX in culture medium caused abnormal elastic fiber assembly. Immunoblotting and immunofluorescence show that LOX and tropoelastin are present together with fibronectin on the cell surface of preconfluent cultures. Further, fluorescence activated cell sorting (FACS) analysis for the localization of LOX on the cell surface reveals that the transfer of LOX to the extracellular space occurs in association with elastic fiber formation. In conclusion, our results support the view that LOX and tropoelastin are present on the cell surface and suggests the possibility that lysine oxidation by LOX precedes tropoelastin deposition onto microfibrils.
Elastic fibers are major insoluble extracellular matrix structures that impart elasticity to organs such as skin, lung, ligament and arteries. Elastin assembly into a fibrillar matrix is believed to be a complex stepwise process. In the first step, secreted soluble monomeric elastin molecules (called tropoelastin) self-aggregate thorough coacervation, a process that concentrates and aligns tropoelastin molecules for cross-linking. Tropoelastin aggregates are then deposited onto preformed microfibrillar templates, which act as a molecular scaffold. Finally, the oxidative deamination of peptidyl lysine residues in tropoelastin is catalyzed by lysyl oxidase (LOX) to form mature elastic fibers. However, the precise mechanism of elastic fiber assembly remains unknown.
Self-aggregation of tropoelastin through coacervation plays an important role in the alignment of tropoelastin monomers for polymeric assembly and cross-link formation.1,2) Hydrophobic domains of tropoelastin are rich in amino acids such as glycine, proline, valine and leucine, which are present in a variety of tandem repeat sequence, i.e., VGVAPG and GGLG(V/A). The tandem repeat sequences aggregate via β-sheet/β-turn structure3,4) and thereby contribute to the polymerization of tropoelastin.5,6) We have found that exons 16 and 30, which encode hydrophobic domains containing tandem repeat sequences, are important for the assembly process.5,7) The absence of these exons results in a failure of elastin to polymerize on microfibrils.
During coacervation, tropoelastin molecules organize into small spheres and the addition of lysyl oxidase facilitates cross-link of these spheres to form fibers.8) We previously reported that tropoelastin added to the conditioned medium of ARPE-19 cells could polymerize to form fibers. ARPE-19 cells express LOX and microfibril components but do not syntheses elastin.9) Moreover, the addition of β-aminopropionitril (BAPN, an inhibitor of LOX enzyme activity) to the culture medium prevents tropoelastin polymerization, suggesting that the small globular forms of elastin grow to macro aggregates, creating progressively larger fibrillar structures via cross-linking of tropoelastin molecules by lysyl oxidase. A similar finding was made with RFL6 cells where BAPN prevented elastin assembly.9–11) Together, these studies indicate that tropoelastin polymerization by LOX is a crucial step in the early stage of elastin assembly. However, little is known about where or when oxidation of tropoelastin by LOX occurs in the assembly process.
LOX is a secreted copper-dependent amine oxidase that belongs to a multigene family consisting of five members (LOX, LOX-like 1–4), among which LOX and LOX-like 1 (LOXL1) are most similar and comprise a subfamily. These proteins contain a highly conserved carboxyl terminus that comprises a copper-binding motif and a lysyl-tyrosyl-quinone (LTQ) cofactor that are required for protein conformation and catalytic activity, respectively. The oxidative deamination of peptidyl lysine residues in tropoelastin is catalyzed by members of the LOX family to α-aminoadipic acid δ-semialdehyde (allysine), which can spontaneously condense with neighboring amino groups or other peptidyl aldehydes to form covalent cross-links such as desmosine or isodesmosine. Targeted gene inactivation of LOX showed that LOX-deficient mice die during the perinatal period with severely disrupted elastic fiber in skin, lung and aorta.12) Conversely, LOXL1-null mice are viable with normal elastic fiber formation but reduced covalent cross-links.13) These observations suggest that LOX and LOXL play different roles in elastic fiber formation. Here, we found that LOX, but not LOXL, enhances elastin assembly through the catalysis of tropoelastin molecules on the cell surface. Our principal aim was to elucidate the relationship between oxidation by LOX and deposition of tropoelastin.
All reagents were purchased from Sigma-Aldrich (St Louis, MO, U.S.A.) unless otherwise noted.
AntibodiesAntibodies used in this study are as follows: anti-human tropoelastin, anti-human fibrillin-1, anti-lysyl oxidase (Sigma-Aldrich for immunoblot or Novus Biologicals (Tokyo, Japan) for fluorescence activated cell sorting (FACS) analysis), anti-LOXL1 (Sigma-Aldrich), anti-V5 (Invitrogen, Carlsbad, CA, U.S.A.), monoclonal anti-HA (Sigma-Aldrich for immunocytochemistry; or Recenttec K.K. (Tokyo, Japan) for immunoblotting), polyclonal anti-HA (MBL (Nagoya, Japan) for immunoblotting and FACS), anti-epidermal growth factor receptor (EGFR), anti-lactate dehydrogenase (LDH) (Cell Signaling Technology Japan K.K., Tokyo, Japan), anti-HSP90 (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) and anti-Fibronectin (Merck Millipore, Billerica, MA, U.S.A.).
Cell Culture and TransfectionNeonatal normal human dermal fibroblasts (NHDF-neo) and adult normal human skin dermal fibroblasts (NHDF-Ad) obtained from TaKaRa BIO (Otsu, Japan). NHDF cells were maintained in MEMα supplemented with 10% fetal bovine serum (FBS), 100U/mL penicillin/streptomycin (Invitrogen) and 1 mM L-glutamine (Invitrogen) at 37°C and 5% CO2. HEK293T cells and Retrovirus packaging cells, PLAT-A (provided by Dr. T. Kitamura, University of Tokyo) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, 1 mM L-glutamine, 1×non-essential amino acids (Invitrogen), 1 mM sodium pyruvate (Invitrogen) at 37°C and 5% CO2. For PLAT-A, the complete medium contained 1 µg/mL puromycin and 10 µg/mL blasticidin (Both from Invitrogen). Various stable transformants of NHDF-neo cells were established by infecting NHDF-neo cells with recombinant retroviruses or lentiviruses. Recombinant lentiviruses were produced by co-transfection of HEK293T cells with the lentivirus expression vector: pLKO.1-hygro which was a gift from Dr. Bob Weinberg (Addgene plasmid# 24150) and ViraPower packaging plasmids mix (Invitrogen) using Fugene HD (Promega). For production of retroviral particles, PLAT-A was transfected with the retroviruses expression vectors (pMXs-puro, which kindly provided by Dr. T. Kitamura, University of Tokyo). Both cells were cultured at 37°C for 24 h post-transfection. After changing the medium, cells were further incubated at 37°C for 24 h, and the viral supernatant was collected and used for infection. NHDF-neo cells were plated on 24-well plate at 24 h before infection, and the medium was replaced with the undiluted viral supernatant with 8 µg/mL polybrene. Two days later, transformants were selected with medium containing 50 µg/mL hygromycin (Invitrogen) or 1 µg/mL puromycin.
Plasmid ConstructionThe short hairpin (sh)RNA sequence used for the study is LOXshRNA#1; CTG CAC AAT TTC ACC GTA TTA, LOXshRNA#2; CGA CAA CCC TTA TTA CAA, LOXL1shRNA#1; CAT TCA CTA CAC AGG TCG CTA, LOXL1shRNA#2; GCA CGT GAA CCC AAA GTA TAT, CTRLshRNA; TAC AAC AGC CAC AAC GTC TAT. All sequences were BLAST-confirmed for specificity. Synthetic DNA encoding shRNA sequences were cloned into pLKO.1-hygro. LOX-HA and LOXL1-HA were constructed by RT-PCR amplification of total RNA isolated from NHDF-neo cells using the following primers; Forward for LOX (5′-TAA GGA TCC GCC ACC ATG CGC TTC GCC TGG ACC GT-3′), Reverse for LOX (5′-CCG CTC GAG TTA AGC GTA ATC TGG AAC ATC GTA TGG GTA ATA CGG TGA AAT TGT GCA GCC-3′), Forward for LOXL1 (5′-TAA GGA TCC GCC ACC ATG GCT CTG GCC CGA GGC AGC C-3′), Reverse for LOXL1 (5′-CCG CTC GAG TTA AGC GTA ATC TGG AAC ATC GTA TGG GTA GGA TTG GAC AAT TTT GCA GTT-3′). Each reverse primer used for these amplifications included the sequence of the HA tag (YPYDVPDVA) and stop codon (underlined). These amplification products were cloned into pMXs-puro vector. To generate V5-LOX-HA, the sequence of the V5 epitope (GKPIPNPLLGLDST) was inserted into LOX behind the signal peptide using a QuickChange site-directed mutagenesis kit (Agilent Technologies, CA, U.S.A.) according to the manufacturer’s recommendations.
RNA InterferenceNHDF-neo cells were transfected with control small interfering RNA (siRNA) (Thermo Scientific, Waltham, MA, U.S.A.) or fibronectin siRNA (Sigma) using Lipofectamine RNA interference (RNAi) Max (Invitrogen) according to manufacturer’s instructions.
ImmunoblottingOverexpressing of LOX-HA or LOXL1-HA cells were cultured for 5 d after confluent and replaced the medium with serum-free medium. After 48 h, the conditioned medium was collected and centrifuged at 2000×g for 5 min. The supernatant was then precipitated by using ammonium sulfate to bring final concentration to 50%. The cells adhering to the culture dishes were extracted with RIPA buffer (25 mM Tris–HCl, pH 7.6, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA) pH 8.0, 1% NP-40). Aliquots of cell lysates and the ammonium sulfate precipitate were boiled in the 1×LDS buffer with 100 mM dithiothreitol (DTT) and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Separated proteins were electrotransferred to polyvinylidene difluoride (PVDF) membranes (Merck Millipore) and probed with primary antibodies. Bound antibodies were detected using horseradish peroxidase-conjugated secondary antibodies (Jackson). Antibody signals were was enhanced with using chemifluorescent methods from Thermo Fisher Scientific.
RT-Quantitative (q)PCRTotal RNA isolated from cultured NHDF-Neo cells using RNAzol RT (Molecular Research Center Inc., OH, U.S.A.). RT-qPCR was performed using a StepOne Real-Time PCR System (Life Technologies Japan, Tokyo, Japan) with a KAPA SYBR FAST One-step qRT-PCR ABI Prism (Kapa Biosystems, MA, U.S.A.), according to manufacturer’s instruction. Primers used in this study are listed in Table 1.
| Primers | Sequence (5′→3′) |
|---|---|
| LOX-Forward LOX-Reverse | CTCTGACGACAACCCTTATTACAAC |
| CTGGGAGACCGTACTGGAAGT | |
| LOXL1-Forward LOXL1-Reverse | CAGCGTGGGCAGCGTGTACC |
| TCAGGGGCATAGGCTGTGCTGG | |
| LOXL2-Forward LOXL2-Reverse | AGGCGGTGCCTACATCGGGG |
| TGGGTCCGATCCCTTGCCCCA | |
| LOXL3-Forward LOXL3-Reverse | TGCCTGCTGTGCAGTTCGTGC |
| CAGCCAGATGCGGCCTGTTCC | |
| LOXL4-Forward LOXL4-Reverse | GCCCCACATGGCCAACTGCC |
| CACCCTCGGCTCCTCTGCCC | |
| FBLN4-Forward FBLN4-Reverse | CCGTCTGCCCGCAGGCATTG |
| TCGTTGACATCCCGGCAGTGCT | |
| ELN-Forward ELN4-Reverse | CCGCTAAGGCAGCCAAGTATGGA |
| AGCTCCAACCCCGTAAGTAGGAAT | |
| FBN1-Forward FBN1-Reverse | CTGCCCACCTGATTTTGAACTG |
| CCAGAGCGGGTATCAACACAG | |
| Fibronectin-Forward Fibronectin-Reverse | CCCATCAGCAGGAACACCTT |
| GGCTCACTGCAAAGACTTTGAA |
Cells grown on 8-well LabTek chamber slides (Thermo Fisher Scientific, IL, U.S.A.) were cooled to 4°C and washed with cold-PBS containing 1 mM CaCl2 and 1 mM MgCl2 (PBSCM). Cells were incubated with primary antibodies on ice for 30 min. The cells were rinsed with cold PBSCM and fixed with 3% paraformaldehyde in PBSCM. The cells were then incubated with donkey anti-mouse Alexa 546 and anti-rabbit Alexa 488 (Invitrogen) diluted in blocking solution that consisted of PBS containing 3% bovine serum albumin (BSA) for 1h. For immunostaining of fibrillin-1, cells grown on 8-well LabTek chamber slides were fixed with cold-Methanol, blocked with blocking solution, and stained with anti-fibrillin-1 antibody and with the following secondary antibodies; mouse and/or rabbit Alexa Fluor 488 and 546. After three washes with washing buffer (PBS containing 1% BSA and 0.05% Tween 20) the slides was embedded with Dapi Fluoromonut-G (SouthernBiotech, AL, U.S.A.), stained with 4′-6-diamidino-2-phenylindole (DAPI) and images were acquired on a Zeiss LSM510 META confocal microscope, an Olympus FV1200 IX83 confocal microscope or an Olympus IX71 fluorescence microscope.
Purification of Recombinant TropoelastinTo generate recombinant tropoelastin with a V5 epitope at the C-terminus (WT-V5), the full-length human elastin cDNA lacking a signal sequence10) was amplified using forward primer 5′-GGA GGG GTC CCT GGG GCC ATT CC-3′ and reverse primer containing V5-epitope sequence (underlined) without the terminal codon. 5′-TCA ACC GGT ACG CGT AGA ATC GAG ACC GAG GAG AGG GTT AGG GAT AGG CTT ACC TTT TCT CTT CCG GCC ACA GCT TTC CC-3′. The product was inserted into a bacterial expression vector pTrcHis-TOPO (Invitrogen). WT-V5 was obtained by overexpression from the plasmid and purified as described previously.10) Purified recombinant protein was resuspended in 1×LDS buffer (Invitrogen) including 100 mM DTT. Samples were separated by SDS-PAGE and subject to Western blot analysis.
Purification of Recombinant Active LOXTo generate the recombinant active-form of LOX (aLOX), LOX-HA plasmids as templates were amplified using forward primer 5′-TAA GGA TCC AGA CGA CCC TTA CAA CCC CTA-3′ and reverse primer 5′-ATT GAA TTC TTA AAT ACG GTG AAA TTG TGA GCC-3′. The products were inserted into a bacterial expression vector pET-21a (Merck Millipore). aLOX were obtained as described.14) Purified recombinant protein was resuspended in 1×LDS buffer including 100 mM DTT. Samples were separated by SDS-PAGE and subject to Western blot analysis.
Determination of Enzyme ActivityThe enzyme activity of LOX and LOXL1 were determined using an Amplex Red-based analysis.15) Briefly, aLOX was diluted to a concentration of 25 µg/mL in reaction buffer (1.2 M Urea, 50 mM Sodium tetraborate, 1 µM CaCl2, pH 8.2) with 10 mM 1,5-diaminopentane as substrate. After incubation for 30 min at 37°C, horseradish peroxidase (4 unit/mL) and 10 µM Amplex Red (Invitrogen) were added. After incubation for 10 min at 37°C, the fluorescence intensity was recorded with excitation/emission wavelength at 563 and 587 nm. For measurement of LOX-HA and LOXL1-HA enzyme activity, HEK293T cells were transfection with LOX-HA and LOXL1-HA. After 72 h, the cells were collected into a lysate buffer (0.25 M Sucrose, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) pH 7.4 and 1% Triton X-100) and then passed through a 26-gauge syringe needle 20 times while on ice. After centrifugation at 9300×g 20 min, the supernatants (10 µg protein) were diluted with reaction buffer with or without BAPN (500 µM).
Treatment with Recombinant Proteins and BAPNFor the time–course experiments, WT-V5 at a concentration of 1 µg/mL was added to the culture medium of 5 d post-confluent NHDF-neo cells grown in 8-well culture slides. At the end of the incubation time, the medium was replaced by complete medium lacking recombinant tropoelastin. Prior to immunocytochemistry, the cells were washed with PBS. For the addition of BAPN and recombinant proteins (WT-V5 or aLOX), cells were cultured in 8-well chamber slide to 70–80% confluence. A mixture of recombinant LOXs (25 µg/mL) and WT-V5 (10 µg/mL) was added for 4 d with or without BAPN (1, 2.5 or 5 mM) for 6 d. The medium were changed every 2 d.
Isolation of Biotinylated Cell Surface ProteinCell surface proteins were isolated using a cell surface protein isolation kit (Thermo Fisher Scientific) according to the manufacturer’s recommendations. Briefly, NHDF-neo stably expressing LOX-HA and LOXL1-HA or NHDF-Ad were grown to about 70% confluency in 100 mm dishes. After 1 d (pre-confluent) or 5 d (confluent), cells were washed with cold-PBS and incubated with a BS3 (C16H18N2Na2O14S2) solution for 30 min at 4°C with gentle shaking. The biotinylation reaction was quenched, and cells were washed with cold-PBS and lysed. NeutrAvidin agaraose was added to the clarified lysates, and the mixture gently rotated for 1h at room temperature. The agarose beads were washed 5 times with RIPA buffer, and retained proteins were eluted with 1×LDS sample buffer containing 50 mM DTT and subjected to Western blot analysis.
FACS AnalysisNHDF-neo cells overexpressing LOX-HA were cultured on 24-well plates until 70–80% confluent (preconfluent), confluent or 3 d post-confluent. The cells were then washed with PBS and detached by 10 mM EDTA/PBS. The cells were incubated with anti-HA antibody for 30 min at 4°C. After washing, the cells were incubated with Alexa 488-conjugated anti-rabbit whole-immunoglobulin G (IgG) antibody (Invitrogen) for 30 min at 4°C. All incubations and washes were performed in PBS containing 2% FBS. The immunoreactivity of the cells was analyzed using a FACS Verse instrument (BD Biosciences, NJ, U.S.A.).
Statistical AnalysisAn ANOVA was used to assess the statistical significance of the data. In vitro experiments were analyzed thereafter using the Tukey–Kramer multiple comparison tests. Differences were considered significant when p<0.05.
To determine whether lysyl oxidase activity is required for elastin assembly, Neonatal dermal fibroblasts (NHDF-neo) cells were treated with or without BAPN for 6 d. Immunostaining with an antibody against human tropoelastin showed that the treatment of BAPN decreased elastin assembly in a dose dependent manner (Fig. 1A). The presence of BAPN did not affect cell proliferation or expression of elastin, fibrillin-1 or LOX (Figs. 1B, C). These results suggest that the deamination of lysine residues on tropoelastin by LOX is involved in not only the cross-linking but also the deposition of tropoelastin.

(A) NHDF-neo cells were treated with BAPN, an inhibitor of LOX, at the indicated concentrations. After 6 d of culture, cells were immunostained with an antibody against human tropoelastin. Scale bar: 100 µm. (B) Growth rate of NHDF-neo cells incubated with or without BAPN (5 mM). Data are the means of triplicate experiments. Error bars, S.E.M. (C) RT-qPCR analysis for NHDF-neo cells incubated with or without BAPN (5 mM) for 2 and 6 d. Data are expressed as each mRNA/GAPDH mRNA relative to the CTRL (2 d) and the means of triplicate experiments. Error bars, S.E.M.
Next, we performed knockdown experiments using shRNA against LOX and LOXL1. The analysis of RT-qPCR and immunoblotting with the antibodies against LOX or LOXL1 showed that two independent sequences against LOX or LOXL1 dramatically decreased the expression of LOX or LOXL1 in NHDF-neo cells, respectively (Fig. 2A). To evaluate elastic fiber formation in these knockdown cells, we performed immunostaining of elastic fibers with the antibody against human tropoelastin. The knockdown of LOX resulted in no elastic fibers in NHDF-neo cells, whereas LOXL1 knockdown cells generated elastic fibers equivalent to the control shRNA viral infected cells (Fig. 2B). Of note, the expression of elastin and fibulin-4 genes slightly increased by LOX lentiviral shRNA#1 or #2, respectively. Other LOX family genes remained unaffected by the viral infections (Supplementary Materials S1A). In addition, the formation of fibrillin-1 microfibrils remained unchanged in these transfectants (Supplementary Materials S1B).

(A) RT-qPCR analysis and immunoblotting for LOX (left panel) and LOXL1 (right panel) in NHDF-neo stably expressing shRNA against LOX, LOXL1 or a non-target sequence (CTRL). The data are expressed as the ratio of each mRNA/GAPDH mRNA relative to CTRL. Error bars, S.E.M. ** p<0.05, N.S.: non-significant, n=3. Two different shRNA sequences against LOX or LOXL1 were used. (B) Immunostaining of NHDF-neo stably expressing each shRNA with the antibody against human tropoelastin. Scale bar: 20 µm
To verify the involvement of crosslinking by copper amine oxidases in the deposition of tropoelastin, we generated NHDF-neo stably expressing HA-tagged LOX (LOX-HA) and LOX-like 1 (LOXL1-HA), a member of LOX family closely related to LOX. Both proteins were expressed and secreted from each cell line (Fig. 3A). Conditioned medium from LOX-HA cells showed bands at 30 and 32 kDa that represent the active form of LOX. Several bands for LOXL1 were detected in the conditioned medium from the cells stably expressing LOXL1-HA. Confocal microscopy showed that both LOX-HA and LOXL1-HA associated with elastic fibers in the extracellular matrix (Fig. 3B). Although, it is difficult to detect the enzyme activity in NHDF-neo, HA-tagged LOX and LOXL1 indeed have normal LOX activity in HEK293T cells (Fig. 3C). Next, we examined elastin assembly in the cells stably expressing LOX-HA and LOXL1-HA using our established in vitro elastic fiber assembly model.10) Purified recombinant V5-tagged tropoelastin (WT-V5) was detected by Coomassie blue staining and Western blot assay using anti-V5. Exogenous V5-tagged tropoelastin was detected using an antibody specific for human tropoelastin (Fig. 4A). To evaluate elastin assembly in the presence of WT-V5, WT-V5 was added at concentration of 1 µg/mL to the cells stably expressing LOX-HA and LOXL1-HA in culture. No V5 staining was detected in cells expressing LOX-HA in the absence of added WT-V5 (Fig. 4B upper panel). Within 60 min after the addition of recombinant tropoelastin, cells stably expressing LOX-HA organized WT-V5 into a mesh-like matrix that consisted of fine, linear fibers together with thicker fibers that spanned long distances within the culture (Fig. 4B). The organization of V5-elastin in cells stably expressing LOXL1-HA or empty vector was visualized as numerous aggregates that began to form visible fibers at 360 min. By 48 h, elastic fibers in the LOXL1-HA cells became indistinguishable from the LOX-HA matrix except for small elastin aggregates that persisted at later time points (Fig. 4C). Importantly, the formation of fibrillin-1 fibers remained unchanged in these overexpressing cells (Fig. 4D).

(A) NHDF-neo stably expressing LOX-HA and LOXL1-HA were cultured as described in methods. Immunoblotting of the cellular protein (Cell lysate) and the conditioned medium (Medium) from NHDF-neo stably expressing LOX-HA, LOXL1-HA and empty vector as control with an antibody against the HA tag. (B) After 5 d of culture, the transfectants were immunostained with antibodies against human tropoelastin and HA tag. Scale bar: 50 µm. (C) The enzyme activity of LOX-HA and LOXL1-HA in HEK293 cells. Immunoblotting of the cellular protein (Cell lysate) and the conditioned medium (Medium) from HEK293T cells overexpressing LOX-HA, LOXL1-HA or empty vector as control with an antibody against the HA tag. (left panel). The enzyme activity of LOX-HA and LOXL1-HA in HEK293T cells. (right panel) was determined using Amplex-Red-based analysis as described in “Materials and Methods” N=3, Bar±S.E.M. * p<0.05, ** p<0.01 (vs. Empty vector), ## p<0.05.
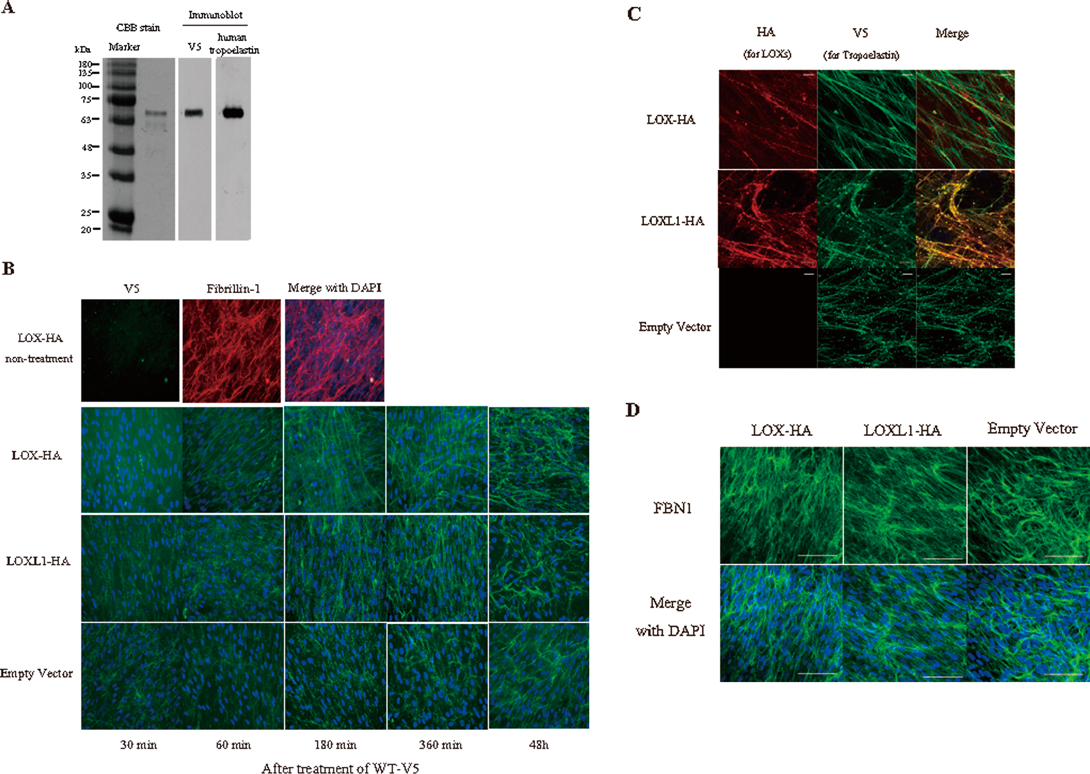
(A) Purified recombinant human tropoelastin with a V5 epitope tag at the C-terminus (WT-V5) was detected by Coomassie blue staining (CBB) and by Western blot using anti-V5 or anti-human tropoelastin antibodies. (B) Cells were treated with WT-V5 (1 µg/mL) for indicated time periods after 5 d post-confluent, followed by immunocytochemistry using the anti-V5 antibody. No staining was detected by anti-V5 antibody without treatment of WT-V5 in NHDF-neo stably expressing LOX-HA (upper panels). Magnification ×200 (C) Confocal laser scanning microscopic observation of colocalizaiton with WT-V5 and LOX/LOXL1 in the transfectants using anti-HA and -V5 antibodies. Scale bar: 10 µm. (D) Immunostaining of the transfectants with antibody against fibrillin-1. Scale bar: 100 µm.
Based on the above results, we assessed elastin assembly when NHDF-neo cells were treated with active LOX in the presence or absence of WT-V5. First, we purified the recombinant active form of LOX (aLOX) using an E.coli expression system (Fig. 5A left panel). The measurement for LOX activity using Amplex red showed that activity of aLOX elevates in a dose-dependent manner (Fig. 5A right panel). Immunostaining of endogenous tropoelastin showed aggregates that accumulated in the extracellular space when NHDF-neo cells are treated with aLOX (Fig. 5B). Immunostaining of NHDF-neo cells treated with WT-V5 only showed the fine network of WT-V5 along with fibrillin-1 positive fibers. When, however, NHDF-neo cells were treated with the mixture of aLOX and WT-V5, massive aggregations of WT-V5 appeared and these aggregations deposited on fibrillin-1 positive fibers (Fig. 5C).

(A) Purified recombinant active form of LOX (aLOX) was detected by CBB and Western blot using anti-LOX antibody. The right panel shows the measurement of enzyme activity of aLOX using Amplex Red method. (B and C) NHDF-neo cells were treated with aLOX (25 µg/mL) in the presence (C) or absence (B) of WT-V5 (10 µg/mL). After 4 d, cells were immunostained with the indicated antibodies. Boxed area in panel is shown enlarged in the right panel. Scale bar: 50 µm (B) or 20 µm (C).
Recently, time-lapse imaging studies showed that tropoelastin aggregates coalesce into linear structures on the cell surface.16,17) Further, previous reports and our present data demonstrated that treatment of pre-confluent cultures with BAPN inhibits elastin assembly.9–11) Therefore, we hypothesized that the deamination of tropoelastin by LOX, an important step in facilitating elastin assembly via tropoelastin polymerization, is occurring on the cell surface. First, to determine the localization of LOX on the cell surface, we isolated and purified cell surface proteins from the cells stably expressing LOX-HA as described in Experimental procedures. Immunoblotting of isolated cell surface proteins using the anti-HA antibody showed that LOX cofractionates at 63 kDa with the EGF receptor as a marker for the plasma membrane, particularly at low cell density (Fig. 6A). We also detected tropoelastin on the cell surface by immunoblotting for biotinylated cell surface proteins (Fig. 6A) and colocalized LOX with tropoelastin by double-immunofluorescence (Fig. 7A). Immunostaining with the antibodies against the HA-tag and EGF receptor also showed that LOX-HA localized on the cell membrane and at the cell edge (Fig. 6B). FACS analysis of the LOX-HA on the cell surface revealed that the number of LOX-positive cells decreased gradually as cell density increased (Fig. 6C). Similarly, localization of LOX on the cell surface was observed in NHDF-Ad (Figs. 6D–F).

(A) Cell surface proteins were isolated from pre-confluent or confluent cells as described Experimental procedures. Immunoblotting was performed using the indicated antibodies. (B) Immunostaining of pre-confluent or confluent cells stably expressing LOX-HA with the antibodies against HA tag and EGFR. Scale bar: 50 µm (Transmitted light), 10 µm (Immunofluorescence). (C) FACS analysis for LOX on the cell surface at pre-confluent, confluent or 3 d post-confluent cultures. A dotted line indicates the negative control. Data are the means of triplicate experiments. Error bars, S.E.M. **, ## p<0.05 vs. pre-confluent. (D) Cell surface proteins were isolated from pre-confluent or confluent NHDF-Ad cells as described in Materials and Methods. Immunoblotting was performed using the indicated antibodies. (E) Immunostaining of pre-confluent NHDF-Neo or NHDF-Ad cells with the antibodies against LOX and EGFR. (F) FACS analysis for LOX on the cell surface at pre-confluent or 3 d post-confluent cultures of NHDF-neo and NHDF-Ad cells. Right panel shows FACS analysis (dot plot) for endogenous LOX on NHDF-neo cell surface. A dotted line indicates the negative control. Data are the means of triplicate experiments. Error bars, S.E.M. **, ## p<0.05 vs. pre-confluent. (F) Double immunofluorescence of LOX and EGFR in NHDF-neo and NHDF-Ad. Scale bar: 10 µm.
LOX has no transmembrane domain and plasma membrane binding proteins for LOX have not been reported. Finally, we investigated how LOX is localized on the cell surface. Cellular-fibronectin strongly bound to LOX18) and the assembly is essential for fibrillin-1 microfibril assembly.19) Therefore, we performed the double immunostaining for fibronectin and LOX when LOX-HA stably expressing cells were transfected with control siRNA or fibronectin siRNA. We found the colocalizaiton of not only LOX with fibronectin on the cell surface but also tropoelastin and fibrillin-1 (Fig. 7A). Further, fibronectin knockdown experiments showed that deficient of fibronectin expression is decreased in localization of LOX on cell surface (Figs. 7B, C).

(A) Double immunofluorescence of tropoelastin, LOX, fibrillin-1 and fibronectin in NHDF-neo stably expressing LOX-HA. Scale bar: 10 µm. (B) NHDF-neo cells stably expressing LOX-HA were transfected with control (CTRL) or fibronectin (FN) siRNA. Left panel shows RT-qPCR analysis for the expression of fibronectin, LOX and fibulin-4 (FBLN4). Right panel shows double immunofluorescence for LOX and fibronectin in the knockdowned cells (right panel). n=3, Error bars, S.E.M. ** p<0.05 vs. CTRL siRNA Scale bar: 10 µm. (C) FACS analysis for LOX on the cell surface at pre-confluent cultures of fibronectin-knockdowned NHDF-neo cells stably expressing LOX-HA. Data are the means of triplicate experiments. Error bars, S.E.M. *, # p<0.05 vs. CTRL siRNA.
LOX initiates the formation of the lysine-derived crosslinks in tropoelastin, resulting in the mechanical stability of elastic fibers. A deficiency in enzyme activity of LOX resulting from treatment with BAPN,20) copper deficient21) or genetic modification12) leads to aortic aneurysms and cardiovascular dysfunction. Previously, we and others found that inhibition of LOX enzyme activity by BAPN leads to loss-of-elastin assembly.9–11) However, little is known about how LOX influences the deposition of tropoelastin. In the present study, we demonstrate that LOX is present on the cell surface and enhances elastin assembly through the catalysis of crosslink formation in tropoelastin at early stage of elastogenesis.
BAPN is a potent irreversible inhibitor of LOX enzymes,22,23) but the mechanism of inhibition of LOX by BAPN has not been defined. In the present study, we demonstrated that BAPN prevents the deposition of tropoelastin by human skin fibroblast similar to what has been shown for rat lung fibroblast.11) Further, a series of knockdown and transfection experiments clearly showed that LOX contributes to the deposition of tropoelastin during elastic fiber assembly via its enzyme catalytic activity. LOXL1, however, plays no role. Histochemical and immunoelectron microscopic analysis of mice with a disrupted LOX gene supports this result; in these animals, elastin is not assembled or is fragmented in skin, lung and aorta.12,24) In contrast, the LOXL1 knockout mouse shows normal deposition of elastin but has defects in elastin regeneration,13) suggesting that LOX is involved in the early stages of elastin fiber assembly and is the major enzyme responsible for catalyzing cross-linking of tropoelastin monomers.
Kozel et al. reported that the deposition of recombinant tropoelastin on pre-existing elastic fibers or microfibrils is independent of LOX enzyme activity.25) Further, in vitro studies using aorta smooth muscle cells and skin fibroblast from LOX deficient mice were able to generate elastic fibers at 9 d after confluency.24) In fact, we also observed similar elastic fiber assembly in LOX-knockdown cells when WT-V5 (10 µg/mL) was added to cells expressing non-target shRNA for 4 d post-confluency (Supplementary Materials S2), suggesting that excessive amounts of tropoelastin are able to deposit on pre-existing microfibrils or elastic fibers regardless of the presence or absence of LOX enzyme activity.
However, in this present study, secreted LOX from cells enhanced the deposition of WT-V5 (1 µg/mL) on pre-existing fibers, creating fine network of fiber structure. FACS analysis revealed that >20% of LOX remained on the cell surface in confluent cultures. And more, the localization of LOX on the cell surface is independent of the presence of tropoelastin, but is promoted by the addition of recombinant tropoelastin as determined by FACS analysis using ARPE-19 cells (data not shown). Therefore, we propose that LOX catalyzes lysine side chains in exogenous tropoelastin on the cell surface, resulting in the promotion of tropoelastin deposition in confluent cultures as well. These results suggest that the cellular/extracellular localization of LOX is important for an orderly elastin assembly.
Fibronectin has been well characterized as an extracellular matrix glycoprotein. Fibronectin is secreted as a dimer bound together by C-terminal disulfide bonds, and it has been traditionally categorized as either soluble plasma fibronectin or insoluble cellular fibronectin (cFN).26–28) Previous report using FN-null mouse embryonic fibroblast indicated that cFN acts as a scaffold protein for LOX and supplies the microenvironments to regulate LOX catalytic activity.18) In this study, we also demonstrate the colocalizaiton of LOX with fibronectin and tropoelastin on cell surface. However, the addition of recombinant aLOX in culture medium result in abnormal elastic fiber formation, indicating that cell-surface localization of LOX through the interaction with cFN is required for elastin assembly. These observations provide new information about the mechanisms of elastic fiber formation, indicating that the interaction of tropoelastin with LOX on the cell surface is an essential process for not only maturation but also elastin assembly.
The authors are grateful to Dr. Yusuke Murasawa, who was very supportive of our experiments. We are also deeply grateful to Prof. Robert P. Mecham, who proofread our manuscript and provided excellent suggestions. We thank Aoi Nakamae and Rieko Ozeki for their technical assistance. This study was supported by a Grant-in-Aid for Young Scientists (B) (F.S.) from the Japan Society for the Promotion of Science (JSPS) KAKENHI.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.